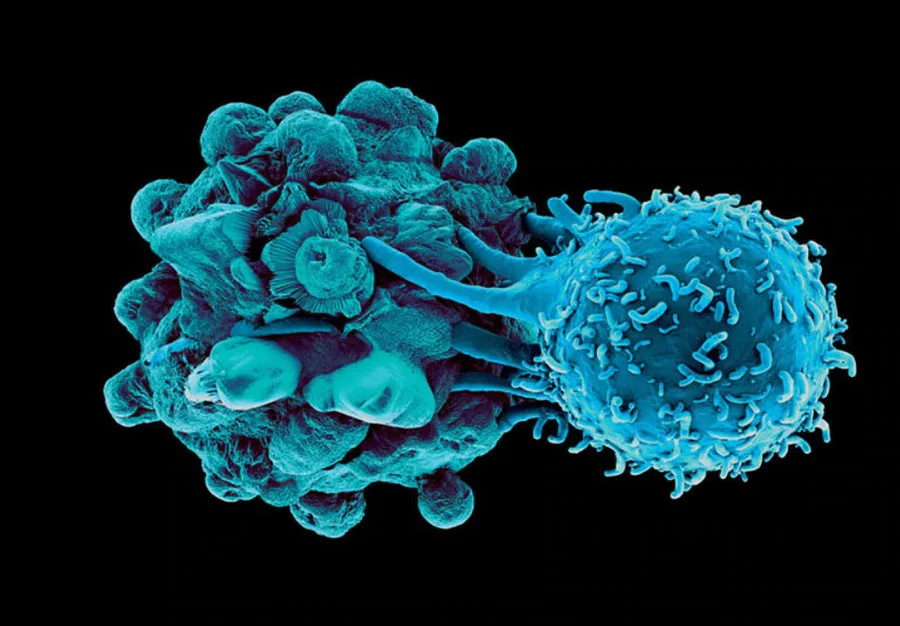
Revolutionizing Melanoma Cancer with Dendritic Cell Treatment
Historically, treatment options for metastatic melanoma have been limited, often relying on chemotherapy, surgery, or radiation therapy, which can have limited effectiveness and significant side effects.
However, advancements in medical research, particularly in that of dendritic cell immunotherapies like ours, have emerged as potential game-changers.
The Future of Melanoma Cancer Treatment is at Immunocine

When it comes to cancer, time is everything. However, for many patients, potentially life-changing treatments remain within the confines of ongoing clinical trials.
Immunocine was founded to bridge this gap. Recognizing the urgent need for advanced treatment options, our sole purpose is to bring a revolutionary immunotherapy to those cancer patients unable to participate in the current clinical trials.
From our base in Cancun, Mexico, we offer our ground-breaking Dendritic Cell Immunotherapy, which is transforming outcomes for patients every day.
Addressing the Challenges Specific to Melanoma Cancer
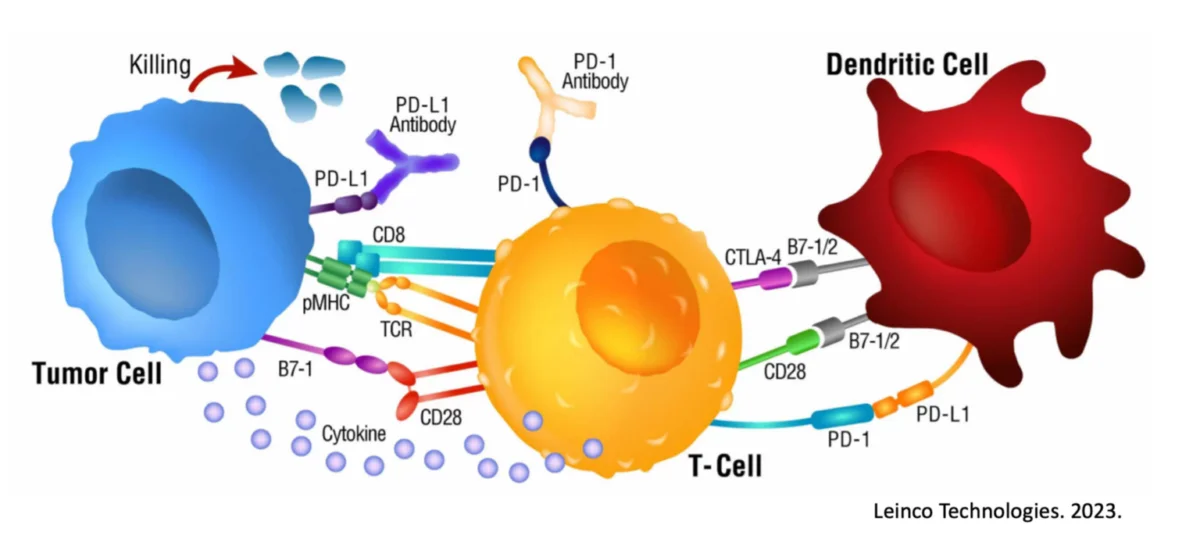
Dendritic cells play a crucial role in initiating and regulating immune responses, including the recognition of cancer cells as foreign invaders. However, in metastatic melanoma, dysfunction of dendritic cells has been observed, impairing their ability to effectively prime T cells and mount an anti-tumor immune response.
Recent studies have shed light on the mechanisms underlying dendritic cell dysfunction in melanoma, including alterations in signaling pathways, immunosuppressive microenvironments within tumors, and interactions with tumor cells and other immune cells.
It has been shown that Dendritic Cell vaccines are able to provide a wide breadth of melanoma antigen targeting to the effector immune cells responsible for targeting and destroying this cancer. This makes it infinitely more difficult for the cancer to be hidden post a vaccine like this, and the immune system finally has an edge. Mathematical models have outlined how a Dendritic Cell vaccine plus checkpoint inhibitor can effectively eradicate tumor cells, a feat witnessed on multiple occasions with Immunocine.

Ease of Metastization
Though each type of skin cancer comes with its own set of challenges, squamous and basal cell carcinomas are typically easy to treat. A majority of patients come to us with melanoma, which can easily metastasize to the liver, lungs, bones, and brain via the circulatory or lymphatic systems.

Genetic Resilience
Melanomas can have high genetic and phenotypic heterogeneity, meaning that different parts of the tumor can display very different genetic mutations and characteristics. Multiple cell subpopulations translate to a higher chance of developing resistance to treatment. Unlike chemicals, the immune system can attack from a new angle.
Taken together, there is reason for optimism when combining the unique robustness and durable immunological memory generated from the Immunocine Dendritic Cell platform with what many have witnessed in general regarding Dendritic Cells and metastatic melanoma.
The scientific rationale plus Immunocine’s own exciting results with patients like Brock, who had over 10 tumors when he arrived for treatment and is now cancer free, further elevate this personalized therapy as a legitimate weapon in overcoming this assiduous cancer.

Our Only Priority is Treating Patients that Will Benefit from Our Treatment

Solid Tumors
While we are constantly expanding our capabilities, right now we only treat patients with solid tumors that can be biopsied by our medical staff. Blood malignancies, such as Leukemia, cannot be treated at this time.

Stable Immune System
For any immunotherapy to be successful, a patient needs to have a stable, functioning immune system. Patients with immune deficiencies and other conditions may not qualify for treatment until normal activity is restored.

Ability to Travel
Some medical conditions and other factors may prevent patients from traveling. Our treatment is administered exclusively at our facility in Cancún, Mexico. If you are unable to travel, you will not be able to receive our treatment.
Six-Week Treatment
Our treatment protocol requires three separate treatments, each two weeks apart. Patients can either choose to stay in Cancun for six weeks or visit twice for two weeks at a time.
Before considering our treatment as an option, please consider the inclusion criteria outlined. In order to be eligible, you must at least meet these requirements.
Documents Required
Medical Records
- Personal medical chart that includes all reports, evaluations, and summaries for the past three to six months.
- All relevant records pertaining to your cancer diagnosis if not included in your personal medical chart.
- Medical imaging reports such as X-Rays, CT Scans, PET Scans, and MRI's.
Recent Blood Panel
- Comprehensive metabolic panel that includes Bilirubin, and AST, ALT.
- Complete blood count with differential and Prothrombin Time (PT) and Partial Thromboplastin Time (PTT).
- If approved, we require a current Hepatitis B, C, and HIV screening.
Are you currently receiving cancer treatment?
Most Immunotherapies are complimentary to our treatment and will not prevent you from qualifying.
However, if you are currently receiving Chemotherapy or Radiation, you will need to wait 30 days after completing those treatments before starting our treatement.
In the mean time, you can still proceed with the Medical Evaluation to see if you would be a candidate once those treatments are complete.
FAQ
We are here to answer any question you may have before or after treatment.
The process begins by collecting a sample of your tumor (“the target”) and your own Dendritic Cells — the “master regulators” of the immune system. In our certified lab, we educate these cells using our proprietary “Double Loading” method, then reintroduce them into your body through the lymphatic system, precisely where immune responses are coordinated.
Because these are your own cells, trained to recognize your own cancer, the treatment is both personalized and biologically derived. Our approach aims to help your immune system identify cancer cells.
Others have focused on what to add to these cells — tumor fragments, RNA, bacteria, toxins — hoping something triggers a response. But we discovered it’s not just about what you give them, but how you give it. Our proprietary Double Loading technique introduces the cancer’s full message in a way that activates Dendritic Cells the way nature intended.
Think of it like having the right key — it’s useless unless you insert it into the lock the right way. Our method flips both immune activation “switches,” leading to results that go far beyond traditional approaches. It’s not a marginal improvement — it’s a qualitative leap.
We also time our cell administration based on immune kinetics, deliver them where the immune system operates best, and combine it all with scientific precision and compassionate care. No one else is doing all of this — together.
Dendritic Cells, when trained correctly, can identify and mobilize against any abnormal cell. Whether your cancer started in the breast, prostate, pancreas, or elsewhere, the immune system doesn’t care about labels — it cares about threats.
We’ve seen success across a wide range of cancers and mutations. And because we’re working with the full picture — not one marker or receptor — our results aren’t limited by subtype. The immune system is designed to protect the entire body. We just help it remember how.
Unlike some clinics that cite generic immunotherapy research, we only reference studies directly tied to our specific method. You can view these on our Research page.
Checkpoint inhibitors remove immune system “brakes.” We re-educate your immune system from scratch — using your own cancer to teach it what to attack. It’s not a passive hope that your immune system will notice the cancer. It’s an active lesson plan, using your cancer as the textbook.
We also:
• Use your entire tumor’s mRNA and protein library — not just one target.
• Deliver immune instructions using our unique Double Loading technique.
• Time and direct the delivery based on immune kinetics and trafficking rules.
• Work regardless of PD-L1 status or prior treatments.
Your immune system likely isn’t broken — it’s just never been properly introduced to the problem. That’s our specialty.
Following our treatment, some patients have shown changes in their cancer’s PD-L1 expression, potentially creating new therapeutic opportunities. Why? Because their immune system is finally fighting back, and the cancer is mounting a defense.
This immune activity causes the cancer to “throw up shields” — like PD-L1 — which weren’t needed before because it wasn’t under attack. That’s when checkpoint inhibitors may suddenly become effective.
We’re not just treating cancer — we’re changing how your immune system sees it, which can unlock future therapies.
• Fatigue (most common)
• Flu-like symptoms
• Muscle aches
• Swollen lymph nodes
• Headaches or nausea
• Localized tumor pain (due to inflammation)
In rare cases, stronger symptoms may occur. Our patients receive treatment in a hospital-grade facility with emergency capabilities, and our team is fully prepared to manage complications if they arise.
Important notes:
• Side effects often peak 24–72 hours after Dendritic Cell administration.
• They may linger for weeks or months as your immune system continues fighting.
• Our treatment protocol includes careful monitoring and preventive measures to manage potential complications.
• Swelling or inflammation on imaging
• Spikes in tumor markers or ctDNA
• New lesions appearing as immune clusters grow
Pseudoprogression is a common occurrence during immunotherapy treatment, and may be observed for several months as part of the immune response. It’s a good sign, but it can look like worsening disease — which is why ongoing monitoring with our team is crucial.
Pseudoprogression is more likely if:
• Your symptoms are improving despite “worse” scans
• Only one marker is rising while others improve
• Steroids reduce symptoms (suggesting inflammation, not tumor growth)
We help you interpret these signals correctly — because mistaking immune activity for cancer progression can lead to premature decisions.
Here’s why we chose Cancun:
• We operate from a certified ISO-7 cleanroom lab with strict regulatory oversight.
• Our medical team includes experienced oncologists and interventional radiologists, many trained in both the U.S. and Mexico.
• Care is delivered within a legal compassionate-use framework approved by COFEPRIS.
• We partner with accredited hospitals like Amerimed, fully equipped for cancer care.
• Treatment here is significantly less expensive than it would be in the U.S.
• Patients often combine treatment with rest and recovery in a peaceful, vacation-like setting.
Cancun checks all the boxes: scientific integrity, clinical safety, legal approval, and patient experience.
To begin the process:
1. Fill out the form here
2. A patient coordinator will contact you to explain the treatment and answer initial questions
3. Submit your completed questionnaire and recent medical records (oncology summary, labs, scans)
4. Our medical team will review your case and issue a recommendation: Approved, Declined, or More Information Needed
5. If approved, you’ll have a call with our team to address any final questions and ensure mutual alignment
All of this is completely free of charge to the patient.
If you are approved, you’ll have 2 weeks to formally accept. This doesn’t mean treatment starts within 2 weeks — only that we need confirmation to hold your place. Treatment should typically begin within 6 weeks, unless otherwise advised by our medical team.
If you’re not ready to pursue treatment within the next 2 months, it’s best to wait on medical review until closer to your preferred timeline.
• Select your preferred start date in Cancun
• Receive the following via DocuSign:
o Informed Consent
o Instructions for Infectious Disease Testing
o Payment Instructions / Invoice
• Ensure your passport is valid
• Get introduced to your personal CARE Team, who will guide your ongoing support
• Coordinate with our Cancun-based concierge to plan travel and logistics
• Review and approve your treatment schedule
• Complete payment before arrival, so we can prepare for your care
Required Tests:
1. HIV-1/2 (Antigen & Antibodies)
2. Hepatitis B (HBsAg)
3. Hepatitis C (HCV Antibody)
4. Syphilis (Treponema pallidum, RPR)
5. Chagas disease (Trypanosoma cruzi Antibody)
6. Active Cytomegalovirus (CMV IgM)
7. Active Toxoplasmosis (Toxoplasma IgM)
8. Active Epstein-Barr Virus (EBV IgM)
Why testing early matters:
• Some results take several days
• Positive results may require confirmation, delaying start dates
• Results are valid for ~7 weeks
If any result is positive:
• HIV, Hep B/C, Syphilis, Chagas: We cannot proceed
• CMV, EBV, Toxoplasmosis (IgM): Treatment must wait until infection resolves We can assist with placing test orders through Ulta Lab Tests, which enables you to pay online and choose a nearby lab (such as Quest Diagnostics) for your sample collection. Please note that we do not directly place the orders.
Standard infectious panel testing typically costs $308, though the price may be higher if confirmatory tests are needed based on your initial results.
Not usable if:
• Stored in formalin (standard pathology method)
• Left out at room temperature
• Preserved only for routine genetic testing
Possibly usable if:
• Immediately cryopreserved (frozen under precise lab-grade conditions)
• Quantity and quality are sufficient after in-lab testing
We’re happy to evaluate any stored tissue, but plan on needing a new biopsy to ensure success.
Note: We’re happy to coordinate with your surgical team if they would like to collaborate, but we are not able to manage the logistics directly with hospitals or surgeons.
Cost: $4,800 (non-refundable, partially credited toward future treatment) Proper Preservation Requirements:
• Use of RNAlater or flash-freezing methods
• Immediate refrigeration (2–8°C)
• Minimum 250mg of tissue
• Priority on RNA preservation (e.g., 80/20 split with pathology)
• Avoid any exposure to formalin
Other Requirements:
• Infectious disease tests must be completed within ±3 weeks of collection
• Pathology confirmation of cancer is required
• Your surgical team must complete documentation to approve specimen release
More detailed instructions will be included with the kit and/or provided by email in advance.
Please visit ImmunocinePreserve.com to register today.
While some therapies, supplements, or alternative treatments may be compatible with our treatment protocol, others could potentially interfere with or diminish treatment effectiveness. Patients must disclose all current and planned treatments to our CARE team for assessment and receive written approval before combining any additional therapies with Immunocine treatment.
As for standard oncology treatments:
• In some cases, we may advise completing them before starting with us
• In other cases, we may recommend pausing them during Immunocine treatment
• This will be discussed during your Approval Call
Importantly, Immunocine does not close doors to future treatments — chemo, radiation, clinical trials, or alternative protocols remain available if needed.
Your immune system continues learning and fighting long after treatment. That’s why we built a world-class, integrative aftercare model — a three-phase program called the Optimizing Cancer Treatment Package — to help maintain and enhance your results.
Included in your treatment cost:
Phase 1 (Months 1–3):
• Establish metabolic baseline
• Comprehensive lab analysis
• Custom supplement/nutrition protocols
• Coordination with your local oncologist
• Access to full CARE team
• Full oversight of your cancer strategy
• Metabolic polarization toward immunity
Phase 2 (Months 4–6):
• Immune response monitoring
• Ongoing data review and adjustments
• Personalized interventions as needed
Phase 3 (Long-term):
• Advanced terrain testing
• Genetic, microbiome, and heavy metal assessments
• Strategy for recurrence prevention and long-term vitality
• Focus on ongoing ImmunoMetabolism and Systems Health
Your CARE team includes:
• Dr. Janet Maendel (Integrative Medicine Director)
• Dr. Steven Noga (Integrative Oncologist, Johns Hopkins)
• Dr. Gordon Grado (Radiation Oncology)
• Dr. Matthew Halpert (Immunologist, CSO)
• Dr. Vanaja Konduri (Co-founder, Immunocine tech)
• Penny Daugherty, RN (Oncology Navigator)
• Patricia Moore (Patient Experience Director)
• Elita A-Vard (Metabolic Patient Advocate)
• Continued involvement of Cancun Medical Experts
The takeaway:
We don’t just treat you and send you home — we stay with you. This is a strategic partnership built to optimize your body’s defense system long after you’ve left Cancun.
Phase 1A: Initial Week in Cancun
You’ll spend approximately one week on-site. During this time:
• We conduct baseline scans, lab work, and immunological assessments.
• A biopsy is performed at a certified private hospital (usually outpatient).
• We mobilize your white blood cells and collect them through a blood draw or apheresis procedure.
By the end of this phase, we’ll have your tumor sample and the necessary immune cells to begin creating your personalized therapy.
Phase 1B: Dendritic Cell Preparation (Approx. 1 Week)
You can stay in Cancun or travel while our lab team prepares your custom Dendritic Cell vaccine. If any synergistic treatments are applicable, we may coordinate them during this week. You’ll receive a clear schedule in advance, so there are no surprises.
Phase 2: Dendritic Cell Administration
You’ll return to Cancun (or remain) for three treatment sessions spaced roughly two weeks apart. Each visit lasts about 3 days and includes:
• Lab tests and oncological reviews
• Image-guided administration of your Dendritic Cells
• Immune-enhancing agents, such as Pegylated Interferon Alpha
Between sessions, you’re free to rest in Cancun or travel.
Phase 3: Continued Support at Home
Once you return home, your immune response is typically just beginning. Our CARE team — including integrative oncologists, metabolic advisors, and nurse navigators — will continue working with you. While we can’t replace your local medical care, we’ll provide ongoing support, data review, and optional booster planning.
Many patients later return to Cancun for a final testimonial filming — a meaningful milestone in their journey.
To try and be transparent and reduce stress on the patient, Immunocine operates via a one-time fee model which covers all that goes into production and treatment, with no “ongoing bills,” “black holes,” or dearth of communication that often frustrate patients and their families.
The treatment program fee is $120,000 USD, covering the core medical, scientific and logistical components of your personalized therapy program.
Below is a general overview of what is covered; but a more in-depth look at the relative costs and values can be found here.
This covers:
• All doctors, specialists, lab work, scans, and procedures
• Collection and manufacturing of your personalized Dendritic Cell therapy
• Scientific personnel, reagents, and quality control certifications
• Image-guided administration of activated Dendritic Cells near draining lymph nodes
• Storage of extra Dendritic Cells for potential future boosts
• Immune-enhancing agents (e.g. IFN-α, CpG) when applicable
• Continual and responsive interaction and data review with our medical and advisory team
• A private driver for airport and medical transport
• Full concierge coordination
• Deep dive, patient-specific immunological analytics
There are no hidden fees or a la carte upsells. Once accepted into the program, this is your price.
Not included:
• Airfare or lodging (our team will help arrange both and offer preferred hotel rates)
• Cost of future Dendritic Cell “boosts” ($4,000 per boost, if pursued later)
• Emergency interventions not directly related to the treatment protocol
We also offer a transparent refund policy, which can be further explained below.
1) https://progressreport.cancer.gov/after/economic_burden
2) https://pubmed.ncbi.nlm.nih.gov/39367130/
3) https://www.managedhealthcareexecutive.com/view/the-cost-of-new-cancer-drugs-is-increasing
4) https://www.psu.edu/news/research/story/cancer-costs-us-more-156-billion-drugs-leading-expense
Full Refund:
• 100% if canceled more than 3 weeks before your start date
• 95% if canceled within 3 weeks and no medical procedures have begun
Partial Refunds (Post Arrival):
| Stage of Treatment | Refund Amount |
|---|---|
| Before scans/tests/biopsy | 90% |
| After baseline scans | 80% |
| After biopsy | 70% |
| After apheresis | 50% |
| After first cell injection | 0% |
• Insufficient biopsy or cell sample
• Sudden illness or emergency
• New medical complications
• Immunocine team decision to pause treatment for safety
Due to the complexity and cost of manufacturing the personalized treatment, no refunds can be provided once treatment has commenced with the first injection.
To ensure optimal treatment efficacy and operational efficiency, patients are required to adhere to their scheduled appointments. Multiple missed appointments or consistent delays may necessitate treatment cancellation without refund, as such disruptions impact both treatment effectiveness and facility operations.
LightStream Medical Financing:
• Fixed-rate loans with no collateral required
• Terms of 3–7 years
• Fast approval process, often within 5 days
Life Credit:
• May allow you to borrow against your life insurance policy
• We recommend reviewing terms directly with Life Credit, as Immunocine does not endorse or manage these agreements
Let us know if you’re interested in learning more.
However, we’ve had patients successfully recover significant funds through insurance reimbursement after treatment.
We now partner with a third-party reimbursement group that:
• Specializes in medical tourism
• Works on a contingency basis (no upfront cost)
• Only takes a fee from recovered funds
This team can assist with submitting your documentation post-treatment. While insurance coverage cannot be guaranteed and should not be assumed, some patients have successfully obtained reimbursement. Immunocine makes no representations or warranties regarding insurance coverage
We’ll help you:
• Choose from vetted hotel options (with preferred rates)
• Coordinate airport pick-up and medical transport
• Finalize your treatment and lab schedule in advance
We want you focused on healing — not logistics.
In fact, research shows that about two-thirds of cancer-causing mutations are due to random replication errors, not lifestyle choices or inherited genes.
Normally, your immune system detects and destroys these abnormal cells before they can grow. But in the case of cancer, the immune system missed the signal — a phenomenon known as “immunological ignorance.” The cancer cell essentially flew under the radar and was never flagged as a threat.
That’s where Immunocine comes in.
Our Dendritic Cell treatment doesn’t “boost” your immune system — it educates it. We teach your immune system exactly what it missed so it can finally recognize specific characteristics of cancer cells, potentially enabling it to target these cells more effectively. The goal is to correct the oversight and restore your immune system’s natural ability to protect you.
• It’s constantly changing. Within a single tumor, there may be hundreds of variations of cancer cells, making it hard for treatments to stay effective.
• It suppresses the immune system. Tumors release chemicals and present surface markers (like PD-L1) that actively shut down immune responses.
• It grows fast. Some cancers grow faster than treatments can catch up.
• It hijacks your metabolism. Cancer cells consume massive energy and flood their surroundings with waste that weakens immune cells.
• It’s genetically unstable. Cancer adapts rapidly, developing resistance to drugs.
• It hides in hard-to-reach places. Location can make treatment riskier or less effective.
How we’re different:
Our treatment educates your immune system using the full mRNA (the underlying blueprints) and protein library (the structural representation) of your cancer — not just a single marker. Even as cancer changes, your immune system has been taught to recognize multiple features of the threat. We don’t try to outrun cancer — we give your body the tools to find it, adapt to it, and destroy it.
Think of your immune system as a military force. Most immunotherapies either remove the brakes, give it new weapons, or send it into battle. Immunocine trains it — teaching your immune system who the enemy is and how to fight it.
Here’s how some current FDA-approved immunotherapies work:
Checkpoint Inhibitors (e.g. Keytruda, Opdivo):
• Remove the immune system’s “brakes”
• Work only if your immune system already recognizes the cancer • Effective in 15–25% of patients
• Immunocine Advantage: We teach your immune system to recognize cancer first — potentially making these therapies effective later
CAR-T Cell Therapy:
• Engineers your T-cells in a lab to target one cancer protein
• Costly ($400k+), often ineffective for solid tumors, high side effect risk
• Immunocine Advantage: We target your entire cancer, not just one marker — with less risk of autoimmune sequelae
Monoclonal Antibodies (e.g. Herceptin):
• Lab-made proteins that bind to specific cancer proteins
• Cancer often mutates around them
• Immunocine Advantage: We train the immune system to recognize multiple targets, not just one
Our Dendritic Cell Approach:
• Personalized to your specific cancer
• Uses your entire cancer profile (mRNA + protein)
• Works regardless of biomarkers like PD-L1
• Fewer side effects, biologically aligned with your immune system
• Applicable to most solid tumors
• Less expensive and more accessible than CAR-T
Key takeaway: Most immunotherapies enhance a response. We create one. We don’t just take the brakes off — we give your immune system a map, a mission, and the training to succeed.
Here’s why resistance is less likely:
• Multi-target recognition: We educate your immune system on dozens or even hundreds of unique cancer markers
• Immune memory: Unlike drugs, your immune system remembers what to look for
• Broad pressure: We don’t give cancer a single threat to escape — we surround it
• Natural surveillance: We’re restoring a system evolved over millions of years to protect you
• Dynamic adaptation: Your immune system can evolve alongside the cancer
• Metabolic targeting: We teach your immune system to recognize how cancer behaves, not just what it looks like
Cancer can mutate around a drug. It can’t easily escape an immune system that understands its full identity.
• In our experience, we have observed patients who have remained cancer-free for extended periods following treatment, including one case exceeding 7 years.
• Several others are approaching 5 years with no recurrence
• These aren’t just survival stats — these are full, active lives being lived
Why we believe it lasts:
• Our treatment generates CD8+CD161+ memory T cells — long-lasting immune cells with:
o Superior killing power
o Tissue-homing ability
o Resistance to exhaustion
o Self-renewal capacity
Think of it like installing a permanent security system in your body — one that never clocks out.
Additional safeguards:
• Booster doses can be stored and administered if needed
• Repeat treatment is possible — your immune system can’t develop resistance to itself
• Our approach creates durable immune memory — just like childhood vaccines

Call Us
+1 (888) 575-2572

care@immunocine.com

Fax
+1 (832) 827-4875

Apply for Treatment
Unlock your body’s natural defense against your unique cancer.
Let’s Get Started With Your Treatment
A full medical screening must be conducted prior to qualifying for the Immunocine Dendritic Cell Treatment.
To start the screening process, our team will host an introductory call to provide an overview of the treatment and answer any initial questions that you may have. Your current physcian and family members are more than welcome to join this call.
To get started, please complete this form. Once submitted, a member of our team will reach out to schedule the call.