While every patient’s treatment is unique, many patients have similar questions about our treatment.

Below, we’ve answered frequently asked questions. But, as always, do not hesitate to reach out directly to answer any specific question that you may have about our treatment.
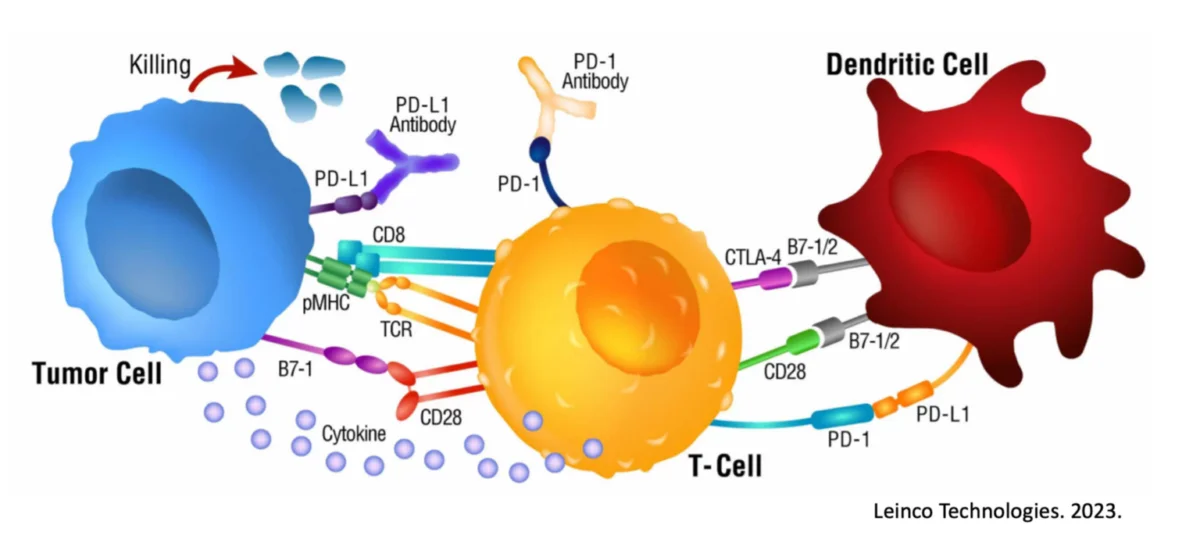
Our treatment starts with collecting both a sample of a patient’s tumor and their own Dendritic Cells. In the Immunocine Laboratory, our team educates these Dendritic Cells to recognize and initiate a comprehensive attack against the cancer cells. Our treatment is completely personalized to each patient and their specific cancer.
As these are your own cells and nothing foreign is introduced to your body, our treatment is actually natural and safe despite being incredibly effective. Our treatment paradigm revolves around your body’s natural ability to destroy the cancer cells if it knows what cells to attack specifically.
Even including other cancer treatments beyond Dendritic Cell Treatments, our treatment is the only treatment to stimulate a TH1 High Response against a patient’s cancer cells.
This is the same response generated during a viral infection and corresponds with the rapid activation of specific immune cells capable of destroying any targeted cell in the body. Our Team does this through a patented process that effectively “Double Loads” the Dendritic Cell, in a very similar manner to an invading virus.
In a viral response, your body’s primary defense is to destroy the virally infected cells before they infect other cells. Instead of targeting the virally infected cells in your body, our treatment enacts the same response but instead targets the cancer cells.
Other than the FDA Clinical Trials for Glioblastoma and Pancreatic Cancer, Immunocine is the only treatment center where you can receive this proprietary Dendritic Cell Therapy.
Peer-reviewed research has proven time and time again that Double Loading is the only known way to fully activate a Th1 immune response, which is necessary to engage a patient’s Killer T cells to destroy the cancer cells. In comparison, single loading only generates a limited immune response.
Treatment Administration – Immunocine’s Treatment is one of the few treatments that use an Interventional Radiologist to administer the treatment via an ultrasound-guided injection. The Interventional Radiologist targets the injection as close as possible to the patient’s lymph node closest to their tumor.
This step is also essential to ensure the Dendritic Cells immediately start activating an immune response. Other Dendritic Cell Treatments rely on intramuscular injection or IV infusion which will delay treatment onset and risk the Dendritic Cells not fully activating the Immune System.
We would be more than happy to share the specific publications with any patient or referring physician interested in learning more
While some patients choose to stay in Cancun for all six weeks, many patients split their stay into two trips. As there are extended periods of downtime to allow the Immune System to respond to the treatment, many patients will come to Cancun for two weeks to start the treatment and return for a two-week trip to complete the treatment.
Patients with tumors in sensitive locations or in their bones may also feel a varying level of soreness at the tumor sites as a result of the immune response.
Fortunately, these symptoms will reduce over time and will disappear once the patient’s cancer has been eliminated. Furthermore, these symptoms are only the result of the Immune System attacking the patient’s cancer and are a positive sign that the treatment is working.
We know that this is a price that may not be accessible to many patients. Unfortunately, each treatment is personalized to every patient and takes over a week to develop in our cGMP laboratory. Without cutting any corners, our team has tried to make the treatment as affordable as possible.
What do I get?
Medical Personnel
Oncologist
Radiologist
General
Internist
Hematologist
Angiologist
Phlebotomists
Nurses
Procedures
US-Guided Injections
of personalized vaccine
Scans and Imaging
Biopsies
Pathology
Central Line
Aphaeresis
Blood Draws
Overnight hospital stay (if needed)
Scientific Personnel
Molecular Biologist
Cell Culture Technologist
Cancer Immunotherapist
Vaccine Development
Reagents
Centrifuges
Incubators
Tissue Hoods
Media and Cytokines
QC Analysis
mRNA Isolation
mRNA Amplification
Lysate Preparation
Sterility
Microscopy
Flow Cytometry
Etc.
Coordination
Patient Concierges
Transportation
Advisory Board
Medications
Neupogen
Pegylated IFNα
Symptom Management
Data Analysis
Radiology Reports
Pathology Reports
Flow Analysis
Blood Lab work
Data Reports
Facilities
CGMP ISO-7 Laboratory
Procedure Rooms
Private, Full-Service Hospital
Imaging Centers
Clinics
Ongoing Care
Oncological Nurse Navigator
Continued Data Review
Coordination with home medical (if desired)
Storage of Boost Dendritic Cells
LightStream Medical Financing – LightStream guarantees the lowest interest rate on Medical Financing. You can choose to pay the loan back in 3 – 7 years with payments lower than $1,300 per month. Your approval does not require any collateral and can be determined in as little as 5 days.
Life Credit – Life Credit will allow you to borrow up to 50% against your life insurance. This allows you to keep your life insurance and any associated benefits.
The Medical Evaluation also gives patients an opportunity to speak with our Medical Team to address any questions they may have or connect our team with the patient’s current healthcare providers.
Once you complete the Preliminary Medical Evaluation to determine your eligibility, our team will be more than happy to connect you with a treated patient of ours.
Once the patient has passed the Preliminary Medical Evaluation, their records are then reviewed by the larger Medical Team, which includes our Oncologist, Radiologist, and Hematologist. During the Final Medical Evaluation, our team will be doing a deeper review of your records and also developing our treatment strategy for the patient.
We complete this process in roughly ten days once we have received all medical records. If approved for treatment, one of our Patient Coordinators will soon be calling to share the good news!
If treatment is declined, a member of our Medical Team will reach out to explain the rationale for the decision and any recommendations they have for future treatment options outside of Immunocine.
Travel to Cancun, Mexico – Our Treatment Protocol must be administered in Cancun, Mexico. Unfortunately, if you are unable to travel to Cancun, you will not be able to receive treatment.
Stable Immune System – For our Immunotherapy to be successful, a patient needs to have a stable, functioning Immune System. Patients with immune deficiencies may not qualify for treatment until normal activity is restored.
Treatment for Six Weeks – Immunocine’s Treatment Protocol takes place over 6 weeks. Patients can either choose to stay in Cancun for all 6 weeks or visit Cancun twice for 2 weeks at a time.
Prognosis Greater than 3 Months – While our treatment has seen success where other treatments have not, our treatment takes time to work fully. Patients with a prognosis of fewer than 3 months may not achieve desired results in such a short amount of time.
Once you are approved for treatment, our team will reach out to answer any travel questions that you may have. In addition, our team is very well-versed in the Cancun area and can provide recommendations on where to stay based on your preferences and needs.
Once in Cancun, all transportation within Cancun will already be arranged with a private driver. You will not need to worry about renting a car or coordinating any logistics for your appointments.
As a note, nursing services are provided at an additional cost and are dependent on the patient’s specific needs. If this is an option you would like to explore, please let us know, and we can provide general cost estimates.
The interactions between treatments may overwhelm the Immune System or may not even be necessary upon completion of our treatment.
If you are a patient that could qualify for these clinical trials, our team will connect you with the US Team responsible for leading the trial recruitment.
This work has resulted in multiple active FDA Clinical Trials studying the efficacy of this treatment. While this represents significant potential for cancer treatment, it did not help patients who did not qualify for the clinical trials.
In 2021, Dr. Halpert founded Immunocine with the sole purpose of bringing this revolutionary immunotherapy to cancer patients unable to participate in ongoing FDA Clinical Trials.
Medical Team – Led by Immunocine’s Chief Medical Officer, Dr. Alberto Ortiz, Immunocine’s Medical Team consists of experienced physicians in Oncology, Interventional Radiology, and Hematology. This complete Medical Team ensures that all patients are evaluated thoroughly and receive the highest level of care upon arriving in Cancun.
Science Team – Led by Matthew Halpert, PhD, and Susana Hernandez, MSc, the Science Team consists of cancer immunologists, molecular biologists, and clinical chemists. This team is responsible for creating and exceeding the quality standards for each personalized Dendritic Cell Treatment.
Patient Support Team – Led by Nicole Verrico, the Patient Support Team is responsible for addressing any questions or needs of our patients before, during, and after our treatment. From helping collect medical records to organizing your travel to Cancun, the Patient Support Team makes sure every patient is able to focus on their treatment and getting better.
As a result of the extensive scientific understanding and training involved, it is a difficult process to outsource and ensure the kind of quality we expect at Immunocine.
Therefore, this treatment is currently limited to the teams overseeing the US FDA clinical trials and our team of doctors and scientists in Cancun. As the protocol moves through the FDA approval system and becomes more widespread, the treatment will become more readily available in different locations.
With that being said, our team does not expect it will be much longer until our treatment becomes much more popular as the clinical trials progress. Additional trials have been recently opened and will start enrollment in the coming future.
No different than being able to recognize foreign viruses and attack them, our bodies can do the same with your cancer cells.
Preclinical studies on the Immunocine Treatment have demonstrated immune responses against 100+ different targets and counting. To date, there does not appear to be a specific genetic mutation or tissue type that renders the immune response impotent.
Cancun is a sophisticated city as a result of its large tourism industry. In addition, it has an extensive medical network that is centered around the world-renowned Galenia Hospital, which is internationally accredited and one of the most advanced hospitals in the world.
Furthermore, our partnership with the private, internationally accredited Galenia Hospital ensures every patient is always within a short drive to a well-staffed, well-equipped, well-maintained medical institution if needed. In the event emergent care is required, Immunocine will make sure you are promptly taken care of.
Our Team will continue to review future medical reports, follow their progress, and offer any assistance we can. This includes collaborating on the next steps with a patient’s primary doctors, which we highly encourage.
Every day our bodies will produce cells that have damaged DNA and therefore have the potential to become cancerous cells. Fortunately, our Immune System is very adept at destroying these mutated cells before they are able to grow and proliferate.
However, in rare instances, our Immune System misses a mutated cell. It is these overlooked mutations that ultimately develop and grow into cancer.
Immunosuppressant – The mutated cells that comprise a tumor have already escaped detection by the Immune System. Cancer cells can go further and even actively suppress immune activity by releasing certain compounds or presenting specific surface markers to turn down an immune response.
Aggressive Growth – Certain cancers demonstrate an aggressive growth that simply can outrun a treatment’s progress, especially treatments that are not specifically attacking the cancer.
Initiates a Complete Immune Response – Dendritic Cells have the ability to initiate a complete immune response. As this is initially triggered in Immunocine’s Laboratory, our treatment avoids Immunosuppressant behavior, such as limiting Dendritic Cell maturation.
Aggressive Response – Upon the first Dendritic Cell Injection, your body will start activating Killer T cells to seek and destroy the cancer cells throughout your body. Your body will do this quickly, no different than when it activates Killer T cells to stop a viral infection from spreading.
Through a variety of methods, Immunotherapy treatments leverage this natural ability to destroy cancer cells in a much safer and more effective way.
In comparison, conventional cancer treatments do not use the Immune System. Instead, the majority provide untargeted treatments that hopefully destroy the cancer faster than it destroys healthy cells.
Checkpoint Inhibitors – Cancer cells can express proteins that are normally used to stop an immune response.
Checkpoint Inhibitors block these proteins and therefore allow the Immune System to detect the cancerous cells. Checkpoint Inhibitors also allow for the Immune System to continue functioning longer.
While Checkpoint Inhibitors are easy to administer, they are limited in that they are not often curative for advanced cancers and typically combined with treatments like Chemotherapy.
CAR-T cell Therapy – Chimeric antigen receptor (CAR) T cell therapy genetically modifies a patient’s T cells in the laboratory to specifically target a unique marker on cancer cells. These cells are then infused back into the patient for treatment.
CAR-T cell therapies are used to treat blood malignancies like Leukemia, as they have mostly lacked success in targeting solid tumors.
Monoclonal Antibodies – Antibodies circulate through the body and attach themselves to cancer cells. Once attached to the antigen, antibodies recruit other immune cells to try and destroy the cancerous cells.
Unfortunately, this approach has been repeatedly attempted with generally underwhelming results.
Dendritic Cell Treatments – Dendritic Cells Treatments use a patient’s own Dendritic Cells to target an immune response against cancer. These treatments are usually accompanied by the sampling of a patient’s tumor to provide a target for the Dendritic Cells.
Due to the tumor sampling and Dendritic Cell extraction, Dendritic Cell Treatments represent one of the more complicated and expensive Immunotherapies.
Immunocine’s Treatment is one of the few Immunotherapies that has successfully demonstrated the creation of Immunological Memory. This is the result of initiating of complete immune response via Dendritic Cells as opposed to “short cutting” an immune response with medications to boost the Immune System or genetically modified immune cells that are not able to initiate memory cells.
Besides treatments like coffee enemas that have specifically been demonstrated to make certain cancers progress faster, the majority of these treatments will certainly not make matters worse.
However, our team has yet to find conclusive evidence that these Alternative Treatments can actually eliminate cancer consistently and repeatedly. This is not due to a lack of trying. There are currently 3,000 different clinical trials focused on Fasting alone.
The reality is that cancer is incredibly difficult to treat and often does not allow for endless attempts to find a treatment that is effective. While there is a possibility a patient could see an Alternative Treatment cure them, the majority of patients will most likely not see that success. Therefore, Alternative Treatments represent quite a large gamble for patients that have limited time to effectively treat their cancer.
Start Your Personalized Treatment

1) Apply to See If You Qualify
If you meet the minimum requirements outlined above and are interested in seeing how our treatment could work for your specific case, please submit your info on our submission form or call us directly at +1 (888) 575-2572.

2) Schedule an Intro Call
Our team loves discussing our treatment with potential patients. We will schedule an intro call to learn about your diagnosis and answer any questions that you may have. Prior to the call, we will share additional info for you to review with your family and physician as you see fit.

3) Undergo Medical Evaluation
After confirming your eligibility, our Medical Team will perform a complimentary medical evaluation. This includes a questionnaire and review of your medical records. Once complete, you can be approved for treatment and we can build a personalized treatment program for you.