Metformin + Losartan: Everyday Medicines That May Help “Warm Up” Cold Tumors for Immunotherapy

Many solid tumors are “cold”—fibrotic, poorly perfused, and hard for T cells to enter—so even breakthrough immunotherapies can stall1. Two well-known, generally safe generics—metformin (for type-2 diabetes) and losartan (an angiotensin-receptor blocker for blood pressure)—have emerging evidence that they can remodel the tumor microenvironment and synergize with immunotherapy2, 3. Early clinical signals (and strong mechanistic data) suggest these drugs may help turn some cold tumors hot4, 5. Patients should always discuss changes with their medical team, but these represent worthwhile and potentially critical conversations.
The Problem: “Cold,” Tough-to-Infiltrate Tumors
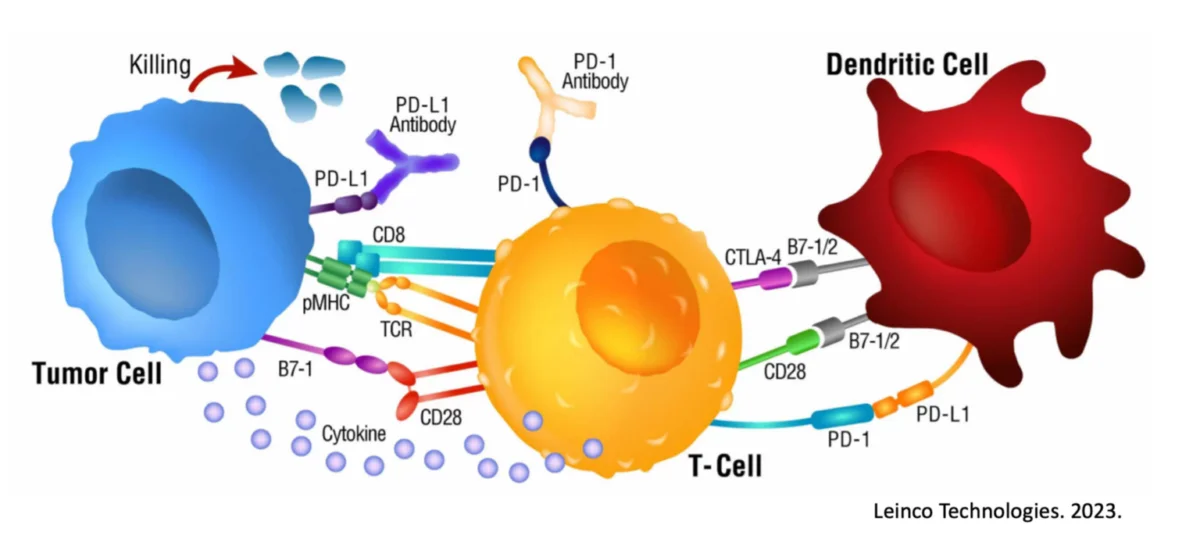
Many cancers build dense extracellular matrix (collagen/hyaluronan), high interstitial pressure, hypoxia, and immunosuppressive myeloid cells6. This combination physically and metabolically excludes T cells and delivered drugs—blunting responses to checkpoint blockade and cell-based immunotherapies7, 8. Strategies that reduce fibrosis, normalize vessels, and relieve hypoxia/metabolic stress can improve drug delivery and T-cell entry—converting tumors from “cold” to “hot.”
Meet the Adjuncts
Losartan (ARB; blood pressure medicine)
- What it is/does: A selective angiotensin II type-1 receptor (AGTR1) blocker used for hypertension and cardiac/renal protection. In tumors, angiotensin signaling in cancer-associated fibroblasts (CAFs) drives collagen deposition and stiffness. Losartan can dampen TGF-β/CAF programs, lower collagen, reduce solid stress, and improve perfusion and immune access9, 10.
Metformin (insulin sensitizer for type-2 diabetes)
- What it is/does: An AMPK activator that lowers hepatic glucose output and improves insulin sensitivity. In cancer settings, metformin can reduce hypoxia, re-program tumor and stromal metabolism, and support CD8⁺ T-cell fitness in low-oxygen niches—mechanisms predicted to enhance checkpoint inhibitor efficacy11, 12, 13.
What the Evidence Shows
Losartan: Human Signals and Mechanism
- Pancreatic cancer (locally advanced neoadjuvant): Adding losartan to FOLFIRINOX, then chemoradiation, yielded notably high R0 resection rates in a single-arm phase II study—consistent with stromal normalization, enabling surgery and therapy delivery. Follow-up correlative work showed down-regulation of collagen/CAF/stiffness programs and microenvironmental changes consistent with improved penetration14.
- Across cancers on immunotherapy: In a multicenter analysis of metastatic renal cell carcinoma, patients taking RAS inhibitors alongside ICIs had significantly longer overall survival and progression-free survival, independent of baseline blood pressure or comorbidities15. Similarly, a 2024 retrospective cohort study in non-small cell lung cancer found ARB use correlated with improved response rates and survival under PD-1/PD-L1 blockade, supporting a mechanistic link between RAS blockade and enhanced immune infiltration16.
Why this matters: By “softening” tumor stroma and reducing solid stress, losartan can increase perfusion and T-cell/therapy access—a key step in warming up cold tumors.
Metformin: Human Signals and Mechanism
- Checkpoint inhibitor cohorts: A multicenter retrospective study (2024) of solid-tumor patients on ICIs found metformin use associated with improved overall and progression-free survival (signal strongest in lung cancer). While retrospective, results align with the drug’s immunometabolic mechanism17.
- Mechanistic human-adjacent evidence: JITC (2023) demonstrated that metformin directly rescues CD8⁺ T-cell function in hypoxic tumor regions, improving antitumor immunity in models that mirror human tumor hypoxia. Reviews summarize multifaceted anticancer mechanisms (angiogenesis, inflammation, metabolism) relevant to human disease13, 18.
Why this matters: By easing hypoxia and improving T-cell bioenergetics, metformin can improve T-cell persistence/function where tumors are most hostile—again, a path to “hotter” tumors under immunotherapy.

Why Pair Them With Immunocine’s IDCT?
IDCT (Immunocine Dendritic Cell Treatment) is a first-in-class dendritic-cell protocol uniquely “double-loading” a patient’s dendritic cells with their own tumor antigens to prime a large, specific killer T-cell response and long-lived immune memory. The goal is potent, systemic anti-tumor immunity19, 20, 21, 22.
- Losartan + IDCT: By reducing stromal stiffness/collagen and normalizing perfusion, losartan may improve trafficking and infiltration of IDCT-primed T cells into desmoplastic tumors (e.g., pancreas, breast), potentially enhancing their on-target killing. Human pancreatic data already show losartan can remodel the microenvironment and enable therapies to reach the tumor2, 14, 22.
- Metformin + IDCT: By reducing hypoxia and supporting CD8⁺ T-cell metabolism in hostile niches, metformin may help sustain IDCT-expanded T cells once they’re inside tumors, complementing dendritic-cell priming with better persistence and function13, 18, 21.
Practical note: Neither drug is a substitute for immunotherapy. But as adjuncts—when not contraindicated—they may help address two core barriers: getting T cells in (losartan) and keeping them active in low-oxygen, suppressive zones (metformin).
Safety, Access, and Cost
Both drugs are generic, widely used, and generally well-tolerated in approved indications. Clinicians already manage their known risks (e.g., renal function and GI effects with metformin; blood pressure, renal monitoring, and potassium with losartan). Importantly, starting either solely for cancer remains off-label, and decisions should be individualized. (Patients should never stop or start medications without their oncologist’s guidance.)

Conclusion
For patients undergoing immunotherapy — including IDCT — and without other contraindications, losartan and metformin represent promising, generally safe, and cost-effective adjuncts to help “warm up” cold tumors: losartan by reducing stromal barriers and improving access, and metformin by supporting T-cell function in hypoxic tumor niches. While prospective randomized trials are still needed, the clinical signals and mechanistic rationale make them reasonable to consider as part of a comprehensive, physician-supervised treatment plan.
References
- Joyce, J.A. & Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015).
- Incio, J. et al. Metformin Reduces Desmoplasia in Pancreatic Cancer by Reprogramming Stellate Cells and Tumor-Associated Macrophages. PLoS One 10, e0141392 (2015).
- Li, X., Luo, X. & Hu, S. Modulating the tumor microenvironment improves antitumor effect of anti-PD-L1 mAb in breast cancer. Bioimpacts 13, 89–96 (2023).
- Sun, Y. et al. Losartan rewires the tumor-immune microenvironment and suppresses IGF-1 to overcome resistance to chemo-immunotherapy in ovarian cancer. Br J Cancer 131, 1683–1693 (2024).
- Wu, Z., Zhang, C. & Najafi, M. Targeting of the tumor immune microenvironment by metformin. J Cell Commun Signal 16, 333–348 (2022).
- Neesse, A., Algul, H., Tuveson, D.A. & Gress, T.M. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 64, 1476–1484 (2015).
- Zeng, W. et al. Myeloid‑derived suppressor cells: Key immunosuppressive regulators and therapeutic targets in colorectal cancer (Review). Int J Oncol 65 (2024).
- Li, L., Li, M. & Jia, Q. Myeloid-derived suppressor cells: Key immunosuppressive regulators and therapeutic targets in cancer. Pathol Res Pract 248, 154711 (2023).
- Chauhan, V.P. et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun 4, 2516 (2013).
- Kim, M.D. et al. Losartan ameliorates TGF-beta1-induced CFTR dysfunction and improves correction by cystic fibrosis modulator therapies. J Clin Invest 132 (2022).
- Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108, 1167–1174 (2001).
- Finisguerra, V. et al. Metformin improves cancer immunotherapy by directly rescuing tumor-infiltrating CD8 T lymphocytes from hypoxia-induced immunosuppression. J Immunother Cancer 11 (2023).
- Zhang, Z. et al. Metformin Enhances the Antitumor Activity of CD8(+) T Lymphocytes via the AMPK-miR-107-Eomes-PD-1 Pathway. J Immunol 204, 2575–2588 (2020).
- Murphy, J.E. et al. Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol 5, 1020–1027 (2019).
- Nuzzo, P.V. et al. Impact of renin-angiotensin system inhibitors on outcomes in patients with metastatic renal cell carcinoma treated with immune-checkpoint inhibitors. Clin Genitourin Cancer 20, 301–306 (2022).
- Mei, J. et al. Angiotensin receptor blocker attacks armored and cold tumors and boosts immune checkpoint blockade. J Immunother Cancer 12 (2024).
- Chiang, C.H. et al. Effect of metformin on outcomes of patients treated with immune checkpoint inhibitors: a retrospective cohort study. Cancer Immunol Immunother 72, 1951–1956 (2023).
- Li, G.Y. et al. Metformin enhances T lymphocyte anti-tumor immunity by increasing the infiltration via vessel normalization. Eur J Pharmacol 944, 175592 (2023).
- Halpert, M.M. et al. MHC class I and II peptide homology regulates the cellular immune response. FASEB J 34, 8082–8101 (2020).
- Konduri, V. et al. Dendritic cell vaccination plus low-dose doxorubicin for the treatment of spontaneous canine hemangiosarcoma. Cancer Gene Ther 26, 282–291 (2019).
- Konduri, V. et al. A subset of cytotoxic effector memory T cells enhances CAR T cell efficacy in a model of pancreatic ductal adenocarcinoma. Sci Transl Med 13 (2021).
- Konduri, V. et al. Chemo-immunotherapy mediates durable cure of orthotopic Kras(G12D)/p53(-/-) pancreatic ductal adenocarcinoma. Oncoimmunology 5, e1213933 (2016).
READ THIS NEXT
The Checkpoint Inhibitor Revolution: Why the First Trials Changed Everything
In March 2011, the FDA approved a drug that would fundamentally change our understanding of cancer treatment forever. Ipilimumab (Yervoy), t
Read MoreOn Air with Immunocine: Matt Halpert Joins Heal Navigator to Discuss How the IDCT is Changing Lives Today
https://www.youtube.com/watch?v=SnFprB1I_K8&list=PLCaoz22dM92-Co9qqZESVpw-GNUdB0MV9 Listen to this Episode on Apple Podcasts Listen to
Read MoreTeam Spotlight: Susana Hernandez, MSc
Meet Susana Hernandez, Immunocine’s Director of Cell Therapy. Based in Cancun, Susana blends deep scientific expertise with a lifelong pas
Read More