Hyperbaric Oxygen Therapy and Cancer Treatment
The relationship between cancer and oxygen is complicated, and nothing written here is going to change that. However, an alternative treatment option sometimes revolves around the use of hyperbaric chambers and ‘oxygen-loading’ programs, and as a matter of due diligence we should dive into the scientific merit here.
Does Oxygen Alter Cancer Progression?
First let us begin with an inherent issue to an aggressive cancer; they cannot get enough oxygen! (1, 2) As a tumor rapidly divides, expands, and grows, it struggles to transport enough nutrients and oxygen to its central areas to keep those cells alive.
The tumor tries, as it executes an angiogenic program (a.k.a. forming new blood vessels to feed itself), but will often fail (3, 4). This is why tumor necrosis (a.k.a. death) can be seen inside some tumors, but is a paradoxical bad sign if there has been no treatment yet (since it really only indicates an aggressive cancer) (5, 6).
Cancer can Adapt
As more and more of the tumor becomes starved for oxygen, it craftily changes its internal programming to utilize hypoxic (a.k.a. oxygen-deficient) pathways and hypoxic inducible factors (HIFs) so as to no longer be at quite a detriment (7, 8). This can also lead to cancers changing the way they metabolize glucose to continue keeping up with energy requirements, and essentially, they adapt to the low-oxygen environment (9, 10).
Unfortunately, a tumor-favoring side effect of being hypoxic is that they become more resistant to cancer treatments, including chemotherapy (11, 12), radiation (13, 14), and immunotherapy (15, 16). All of the exact mechanisms here are still not entirely understood. One dangerous outcome is that HIFs can degrade the matrix surrounding the tumor, allowing for easier escape and metastasis. HIFs can also lead to increased motility of the cells, all feeding into increased cancer aggression.
This and other data points has led to the hypothesis that cancer / tumors “hate” oxygen, and use of something like a hyperbaric chamber (which uses pressure to drive more oxygen into the body) may be beneficial in treating cancer. Is this accurate?
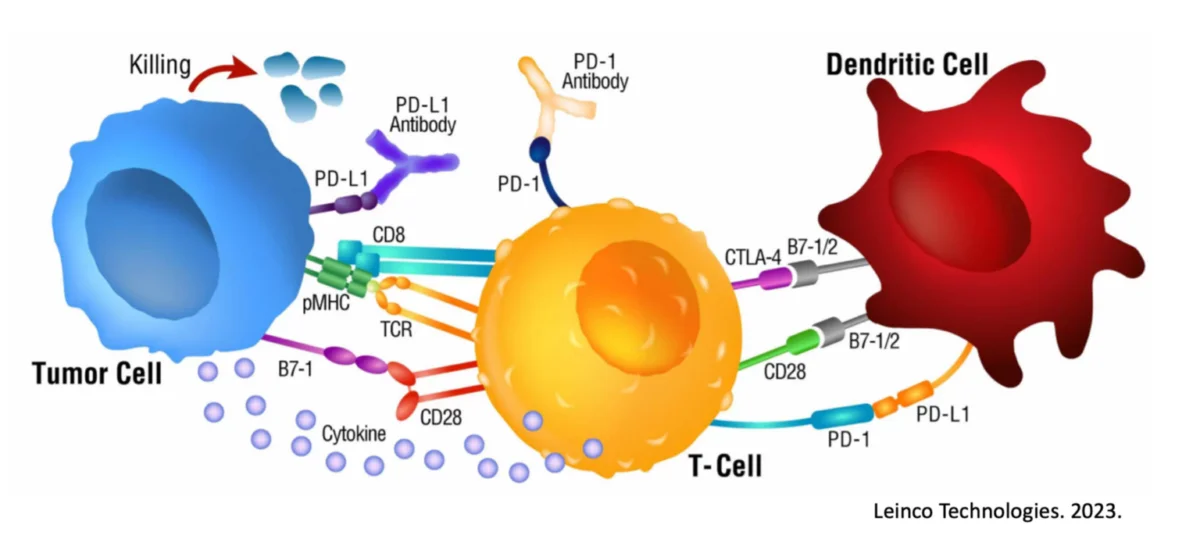
Increased Oxygen Pressure Improves Influx of Activated Immune Cells
At this time, the answer might be “sort of.” In 2014-2015, there was a surge of interest in this treatment modality when some scientific publications started to really hone in on the notion of combining oxygenation with other cancer treatment options. The authors of one such study theorized that there may be an opportunity to provide respiratory hyperoxia (e.g., more oxygen) to reprogram the very immunosuppressive environment of the tumor (a.k.a. tumor microenvironment), based on previous work in which oxygenation did appear to reduce HIF expression within a tumor (17, 18). In fairly straight forward animal work, 60% hyperoxygenation delayed tumor growth and led to upregulation of MHC Class I on tumor cells, which aids in immune-driven destruction. This was found to be somewhat dependent on the presence of T and NK cells, and a secondary cancer treatment arm (17, 18). The authors themselves reported:
“It must be emphasized that our data indicate that there will be no antitumor effects of oxygenation in the absence of antitumor T and NK cells. Therefore, oxygen should be used in combination with other cancer immunotherapies. This message must be very clear to medical professionals and the public since it is a very seductive notion that simply breathing oxygen will cause tumor rejection in any cancer patient.
However, some individual cancer patients may still benefit, such as those who spontaneously develop antitumor T cells or perhaps patients with tumor-targeting immune cells induced by chemotherapy. The identification of such patients with tumor-reactive T and NK cells will be a vital aspect of oxygen therapy.”
Unfortunately, this point about combined efforts is often missed and some have chosen to run with the idea that a cancer patient only needs a hyperbaric chamber to overcome their cancer.
Others have shown that while the use of pressurized oxygen might be useful in at least delaying tumor growth, a review of the studies outlays that different tumors respond differently and underlying reasons are still not understood (19).
However, this may be somewhat dependent on the presence of anti-tumor immune cells within the tumor microenvironment and bears more study.
In a recent study, it was demonstrated that reversing oxygen deficiency within a tumor allowed for upregulation of internal STING signaling and Interferon Regulatory Factor 3 (IRF3) expression (20). Why does this matter? Because this expression on tumor cells was directly attributable to the recruitment of activated CD8 Killer T cells, the same type of cells a good immunotherapy protocol will activate (21).
This combinatorial ‘boost’ has been shown in other scientific studies as well (22).
Though we are effectively still in an infancy regarding the study of hyperbaric oxygenation and immunotherapy capabilities, it would seem potentially advantageous to add this modality after a proper immune response has been educated and activated.
References
1. Muz, B., de la Puente, P., Azab, F. & Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl) 3, 83-92 (2015).
2. Hompland, T., Fjeldbo, C.S. & Lyng, H. Tumor Hypoxia as a Barrier in Cancer Therapy: Why Levels Matter. Cancers (Basel) 13 (2021).
3. Lugano, R., Ramachandran, M. & Dimberg, A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 77, 1745-1770 (2020).
4. Saba, N.F. et al. Targeting Angiogenesis in Squamous Cell Carcinoma of the Head and Neck: Opportunities in the Immunotherapy Era. Cancers (Basel) 14 (2022).
5. Liu, Z.G. & Jiao, D. Necroptosis, tumor necrosis and tumorigenesis. Cell Stress 4, 1-8 (2019).
6. Bredholt, G. et al. Tumor necrosis is an important hallmark of aggressive endometrial cancer and associates with hypoxia, angiogenesis and inflammation responses. Oncotarget 6, 39676-39691 (2015).
7. Wicks, E.E. & Semenza, G.L. Hypoxia-inducible factors: cancer progression and clinical translation. J Clin Invest 132 (2022).
8. Wu, Q. et al. Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape. J Hematol Oncol 15, 77 (2022).
9. Lin, X., Xiao, Z., Chen, T., Liang, S.H. & Guo, H. Glucose Metabolism on Tumor Plasticity, Diagnosis, and Treatment. Front Oncol 10, 317 (2020).
10. Frezza, C. Metabolism and cancer: the future is now. Br J Cancer 122, 133-135 (2020).
11. Belisario, D.C. et al. Hypoxia Dictates Metabolic Rewiring of Tumors: Implications for Chemoresistance. Cells 9 (2020).
12. Doktorova, H., Hrabeta, J., Khalil, M.A. & Eckschlager, T. Hypoxia-induced chemoresistance in cancer cells: The role of not only HIF-1. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 159, 166-177 (2015).
13. Bouleftour, W. et al. A Review of the Role of Hypoxia in Radioresistance in Cancer Therapy. Med Sci Monit 27, e934116 (2021).
14. Rakotomalala, A., Escande, A., Furlan, A., Meignan, S. & Lartigau, E. Hypoxia in Solid Tumors: How Low Oxygenation Impacts the “Six Rs” of Radiotherapy. Front Endocrinol (Lausanne) 12, 742215 (2021).
15. Noman, M.Z. et al. Hypoxia: a key player in antitumor immune response. A Review in the Theme: Cellular Responses to Hypoxia. Am J Physiol Cell Physiol 309, C569-579 (2015).
16. Pietrobon, V. & Marincola, F.M. Hypoxia and the phenomenon of immune exclusion. J Transl Med 19, 9 (2021).
17. Hatfield, S.M. & Sitkovsky, M. Oxygenation to improve cancer vaccines, adoptive cell transfer and blockade of immunological negative regulators. Oncoimmunology 4, e1052934 (2015).
18. Hatfield, S.M. et al. Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1alpha-dependent and extracellular adenosine-mediated tumor protection. J Mol Med (Berl) 92, 1283-1292 (2014).
19. Moen, I. & Stuhr, L.E. Hyperbaric oxygen therapy and cancer–a review. Target Oncol 7, 233-242 (2012).
20. Li, K. et al. Hyperbaric oxygen facilitates teniposide-induced cGAS-STING activation to enhance the antitumor efficacy of PD-1 antibody in HCC. J Immunother Cancer 10 (2022).
21. Konduri, V. et al. CD8(+)CD161(+) T-Cells: Cytotoxic Memory Cells With High Therapeutic Potential. Front Immunol 11, 613204 (2020).
22. Liu, X. et al. Hyperbaric Oxygen Boosts PD-1 Antibody Delivery and T Cell Infiltration for Augmented Immune Responses Against Solid Tumors. Adv Sci (Weinh) 8, e2100233 (2021).
READ THIS NEXT
Overcoming a Rare & Aggressive Kidney Cancer with Immunocine: Justin’s Story
In the prime of his life, Justin was living the life many dream of — raising three young children with his wife and staying in peak physic
Read MoreOn Air with Immunocine: Matt Halpert Joins Haylie Pomroy to Discuss the Future of Cancer Treatment
Listen to this Episode on Apple Podcasts Listen to this Episode on Spotify In this episode of the Hope and Help for Fatigue and Chronic Illn
Read MoreLife After Cancer: Kevin’s Journey with Stage 2C Melanoma Cancer
Kevin, an avid dirt biker and owner of a excavation company operating heavy machinery, faced his toughest challenge not in the wild but in a
Read More