What Is Immunotherapy?
In the late 1800’s Dr. William Coley stumbled onto an interesting observation, that an active immune response in the area of a tumor could delay and even occasionally eliminate a cancerous threat1, 2.

Though he didn’t fully understand the mechanisms at that time, he essentially demonstrated the power of the immune system even against a threat as tough as cancer. And though these “toxins” were occasionally able to generate a successful response, the underpinnings were still obscure and not practical to mainstream medicine. This left the door open for simpler paradigms, such as chemotherapy and radiation, to lead the efforts in the fight against cancer3. Though rife with failure and collateral damage, these remain the leading options even today.
Immunotherapy and the Immune System
Although it took roughly 100 years, utilization of the immune system against cancer began to garner more attention as A) chemotherapies continued to struggle against a variety of diseases and B) the scientific field had gained a better understanding of the cells and mechanisms powering the immune response. Within the first decade of the 2000s, a couple of pivotal clinical trials in which nothing more than removing a “brake” that turns off an immune response was pharmacologically blocked – raising the survival rate from 0% (with chemo) to about 5-10% in very fatal cancers – reinvigorated the field4, 5. This may initially seem disappointing and low until one realizes the promise held within such a result. If a non-specific immune system “booster” could do in 10 years what chemotherapy had been unable to do in 100, then the sky is the limit as we continue to study and understand the true power of our immunity. The immune system can fight back and win against cancer. Paradigms shift.
Types of Immunotherapy: Vaccines, Monoclonal Antibodies, Dendritic Cells, and More
It is important to understand that “immunotherapy” is a broad category encompassing all that angles to educate and empower a patient’s own immune system to destroy cancerous tissue. There is not one type any more than there is one type of chemotherapy or one type of “medicine.” Some of the more common types of immunotherapy today include checkpoint inhibitors, CAR-T cells, antibodies, and Dendritic Cell Vaccines.
Checkpoint Inhibitors
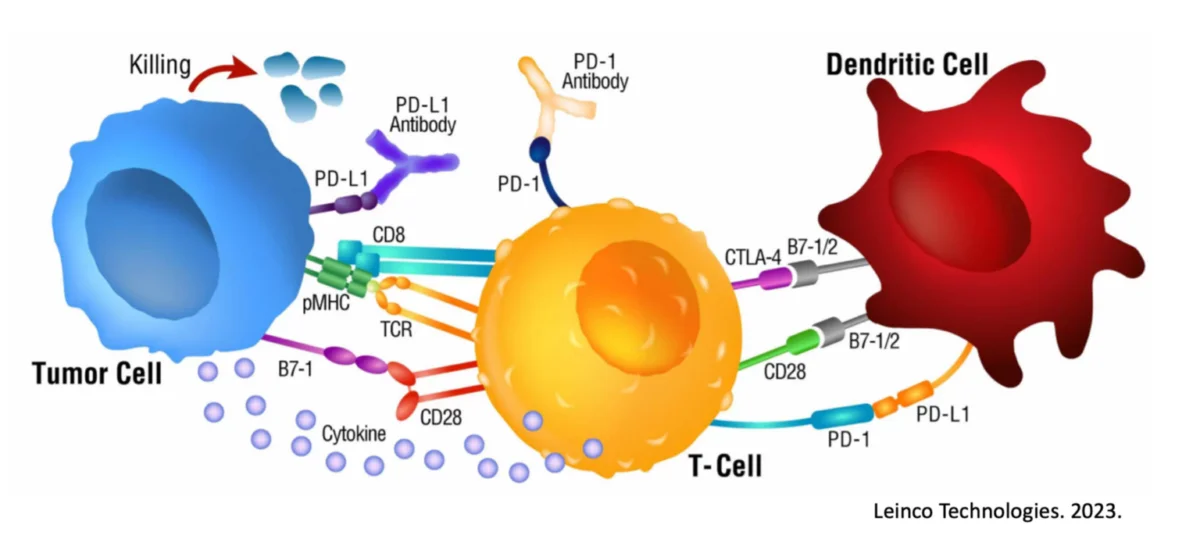
Checkpoint inhibitors, such as against “immune breaks” CTLA-4 and PD-1, interfere with a tumor’s ability to dampen an attacking immune response. By being effectively blocked from pressing the immune cell “off” switch, the immune response can act longer and stronger6, 7.
Pros
- Easy to administer / Off-the-shelf
- Applicable to a variety of cancers
- Relatively safe
- Can delay tumor progression, potentially reversing and in some cases eliminate
Cons
- Not personalized to a patient or a patient’s cancer
- Non-specific in attack; fails to educate the immune system
- Limited half-life and limited efficacy
- Cancer is able to evolve and render useless 8,9
- The potential for long term toxicity issues 10,11
CAR-T Cells
CAR-T cells are a patient’s own T cells that have been genetically modified in the laboratory to “shortcut” the natural immune system and engender a Killer T cell response against a specific marker expressed on the tumor.
Pros
- Able to target a patient’s Killer T cells against a cancer
- Is more personalized than checkpoint inhibitors, but less than Dendritic Cell Vaccines
- Can be self-perpetuating and ongoing
- Integrates into a natural immune response against hematological malignancies
- Can delay cancer progression, potentially reversing and in some cases eliminate
Cons
- Tends to be too weak to overtake solid cancers 12,13
- Typically, only targets a single antigen expressed 14 which can be downregulated rendering the treatment worthless or can be expressed on normal cells leading to off-target destruction
- Not adaptive; does not follow normal pathway for an immune response
- Can cause off-target toxicity 15,16
- Very expensive 17,18
Antibodies
Antibodies are drugs that essentially have 2 functional ends, 1 end targets something expressed on the cancer and the other end recruits immune cells to the site in hopes of introducing an immune attack.
Pros
- Easy to administer / Off-the-shelf
- Applicable to a variety of cancers
- Relatively safe
- Can delay cancer progression, potentially reversing and in some cases eliminate
Cons
- Generally, does not incite a very strong immune response 19,20
- Not personalized to a patient or a patient’s cancer 21
- Non-specific in attack; fails to educate the immune system 22
- Limited half-life and limited efficacy
- Cancer is able to evolve to avoid and render useless 23,24
- Typically, only targets a single antigen expressed 25 which can be downregulated rendering the treatment worthless or can be expressed on normal cells leading to off-target destruction
Dendritic Cell Vaccines
Dendritic Cell Vaccines are the attempt to utilize the natural immune system pathways to generate a global, multi-faceted, and ongoing immune response capable of widespread surveillance and destruction of all cancerous cells.
Pros
- Applicable to a variety of cancers 26,27
- Relatively safe 28,29,30
- Can delay cancer progression, potentially reversing and in some cases eliminate 30,31,32,33
- Is the most personalized immunotherapy 34
- Can be self-perpetuating and ongoing 35,36,37
- Mimics a natural immune response from the “top-down” 28,39
- Leads to the most global immune response 40
- Adaptive; can target multiple antigens expressed on the cancer making it tougher for the cancer to “hide” 41
- Can reduce expression of “brakes” on immune cells to overcome immunosuppressive attempts by tumor microenvironments 42,43
- Most likely immunotherapy to generate immunological memory to help fight against relapses 37
Cons
- Not off-the-shelf; not 1-size fits all
- An expensive process made for each patient
- Previous attempts failed to fully activate Dendritic Cells which led to limited activity
- Depending on protocol, can require a fresh sample of the patient’s tumor
- Has not demonstrated success against hematological malignancies
- Does not delay or reduce tumor burden while immune system ramps up
Immunotherapy In Clinical Trials
Since showing effectiveness in treating cancer, there have been a variety of different clinical trials launched to continue to study the impact that may be possible with immunotherapy. Within the past decade, over 600 trials have been completed for checkpoint inhibitors alone, with another 1800 studies listed as of today, and this is but one immunotherapy modality44. As successes continue to accumulate across the spectrum, it is expected that this number will grow and eventually overtake chemotherapeutic trials.
Immunotherapy Treatment And Side Effects
As with any treatment, patients have a right to be aware of the potential side effects and what may be expected in general. Every patient is unique and therefore effects experienced can be different between individuals undergoing the same treatment, but there are a few generally shared traits that can and should be discussed.
To begin, most immunotherapies will share common symptoms of an “immune response” which can include things such as fever, chills, muscle aches, fatigue, swollen lymph nodes, swollen tumors, inflammation, nausea, appetite changes, and the sort45, 46. Essentially, the therapy is activating or educating a patient’s own immune system to begin destroying the cancer the same way it would destroy an invading pathogen. And eliminating a pathogen that is beyond the introductory stage usually is accompanied with symptomatic evidence of an immune response. Are these side effects? Or rather just the effects of a functioning immune system finally attempting to fight back the cancer?
As a note, within many trials and publications these symptoms may be classified as “Adverse Events (AEs),” which can sometimes scare people who imagine AE’s must refer to things such as stroke, paralysis, and death. AEs are extremely important to track to understand how dangerous a treatment option may be, and until any particular event (a.k.a. symptom) is linked to a positive clinical outcome, it must be considered “Adverse.” But a runny nose can be considered adverse, and this alone should not deter patient’s from exploring these treatment options.
Other than these “natural” symptoms or side effects, the rest of the profile may boil down to the specific type of immunotherapy and to any underlying condition a patient may present with. For example, immune checkpoint inhibitors tend to boost the entire immune response and not just a response against the cancer. This can lead to off-target inflammation and truly erroneous outcomes. However a therapy such as Dendritic Cell Vaccine tends to be far more targeted and specific and thus off-target effects are minimal. This is not to imply there are no risks at all, but as with any treatment option at this juncture, the benefits must be weighed against the risk potential.
References
1. McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J26, 154-158 (2006).
2. Hoption Cann, S.A., van Netten, J.P. & van Netten, C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad Med J 79, 672-680 (2003).
3. DeVita, V.T., Jr. & Chu, E. A history of cancer chemotherapy. Cancer Res 68, 8643-8653 (2008).
4. Alexander, W. The Checkpoint Immunotherapy Revolution: What Started as a Trickle Has Become a Flood, Despite Some Daunting Adverse Effects; New Drugs, Indications, and Combinations Continue to Emerge. P T 41,185-191 (2016).
5. Hodi, F.S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363, 711-723 (2010).
6. Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 11, 3801 (2020).
7. Jacob, J.B., Jacob, M.K. & Parajuli, P. Review of immune checkpoint inhibitors in immuno-oncology. Adv Pharmacol 91, 111-139 (2021).
8. Zaretsky, J.M. et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 375, 819-829 (2016).
9. Barrueto, L. et al. Resistance to Checkpoint Inhibition in Cancer Immunotherapy. Transl Oncol 13, 100738 (2020).
10. Johnson, D.B., Nebhan, C.A., Moslehi, J.J. & Balko, J.M. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol 19, 254-267 (2022).
11. Spiers, L., Coupe, N. & Payne, M. Toxicities associated with checkpoint inhibitors-an overview. Rheumatology (Oxford) 58, vii7-vii16 (2019).
12. D’Aloia, M.M., Zizzari, I.G., Sacchetti, B., Pierelli, L. & Alimandi, M. CAR-T cells: the long and winding road to solid tumors. Cell Death Dis 9, 282 (2018).
13. Marofi, F. et al. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res Ther 12, 81 (2021).
14. Sterner, R.C. & Sterner, R.M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 11,69 (2021).
15. Zhang, Y. et al. Single-Cell Analysis of Target Antigens of CAR-T Reveals a Potential Landscape of “On-Target, Off-Tumor Toxicity”. Front Immunol 12, 799206 (2021).
16. Bonifant, C.L., Jackson, H.J., Brentjens, R.J. & Curran, K.J. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 3, 16011 (2016).
17. Lyman, G.H., Nguyen, A., Snyder, S., Gitlin, M. & Chung, K.C. Economic Evaluation of Chimeric Antigen Receptor T-Cell Therapy by Site of Care Among Patients With Relapsed or Refractory Large B-Cell Lymphoma. JAMA Netw Open 3, e202072 (2020).
18. Keating, S.J., Gu, T., Jun, M.P. & McBride, A. Health Care Resource Utilization and Total Costs of Care Among Patients with Diffuse Large B Cell Lymphoma Treated with Chimeric Antigen Receptor T Cell Therapy in the United States. Transplant Cell Ther 28, 404 e401-404 e406 (2022).
19. Cruz, E. & Kayser, V. Monoclonal antibody therapy of solid tumors: clinical limitations and novel strategies to enhance treatment efficacy. Biologics 13, 33-51 (2019).
20. Sun, A. & Benet, L.Z. Late-Stage Failures of Monoclonal Antibody Drugs: A Retrospective Case Study Analysis. Pharmacology 105, 145-163 (2020).
21. McLaughlin, P. et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 16, 2825-2833 (1998).
22. Benavente, S. et al. Establishment and characterization of a model of acquired resistance to epidermal growth factor receptor targeting agents in human cancer cells. Clin Cancer Res 15, 1585-1592 (2009).
23. Ahmad, A. Current Updates on Trastuzumab Resistance in HER2 Overexpressing Breast Cancers. Adv Exp Med Biol 1152, 217-228 (2019).
24. Mishima, Y. et al. The identification of irreversible rituximab-resistant lymphoma caused by CD20 gene mutations. Blood Cancer J 1, e15 (2011).
25. Patel, D. et al. Monoclonal antibody cetuximab binds to and down-regulates constitutively activated epidermal growth factor receptor vIII on the cell surface. Anticancer Res 27, 3355-3366 (2007).
26. Calmeiro, J. et al. Dendritic Cell Vaccines for Cancer Immunotherapy: The Role of Human Conventional Type 1 Dendritic Cells. Pharmaceutics 12 (2020).
27. Yu, J., Sun, H., Cao, W., Song, Y. & Jiang, Z. Research progress on dendritic cell vaccines in cancer immunotherapy. Exp Hematol Oncol 11, 3 (2022).
28. Nava, S. et al. Safe and Reproducible Preparation of Functional Dendritic Cells for Immunotherapy in Glioblastoma Patients. Stem Cells Transl Med 4, 1164-1172 (2015).
29. Anguille, S., Smits, E.L., Lion, E., van Tendeloo, V.F. & Berneman, Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol 15, e257-267 (2014).
30. Rob, L. et al. Safety and efficacy of dendritic cell-based immunotherapy DCVAC/OvCa added to first-line chemotherapy (carboplatin plus paclitaxel) for epithelial ovarian cancer: a phase 2, open-label, multicenter, randomized trial. J Immunother Cancer 10 (2022).
31. Caro, A.A., Deschoemaeker, S., Allonsius, L., Coosemans, A. & Laoui, D. Dendritic Cell Vaccines: A Promising Approach in the Fight against Ovarian Cancer. Cancers (Basel) 14 (2022).
32. Konduri, V. et al. Chemo-immunotherapy mediates durable cure of orthotopic Kras(G12D)/p53(-/-) pancreatic ductal adenocarcinoma. Oncoimmunology 5, e1213933 (2016).
33. Konduri, V. et al. Dendritic cell vaccination plus low-dose doxorubicin for the treatment of spontaneous canine hemangiosarcoma. Cancer Gene Ther 26, 282-291 (2019).
34. Gardner, A., de Mingo Pulido, A. & Ruffell, B. Dendritic Cells and Their Role in Immunotherapy. Front Immunol11, 924 (2020).
35. Hole, C.R. et al. Induction of memory-like dendritic cell responses in vivo. Nat Commun 10, 2955 (2019).
36. Silva, M.O. et al. Antigen Delivery to DEC205(+) Dendritic Cells Induces Immunological Memory and Protective Therapeutic Effects against HPV-Associated Tumors at Different Anatomical Sites. Int J Biol Sci 17, 2944-2956 (2021).
37. Zammit, D.J., Cauley, L.S., Pham, Q.M. & Lefrancois, L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity 22, 561-570 (2005).
38. Halpert, M.M. et al. MHC class I and II peptide homology regulates the cellular immune response. FASEB J 34,8082-8101 (2020).
39. Qian, C. & Cao, X. Dendritic cells in the regulation of immunity and inflammation. Semin Immunol 35, 3-11 (2018).
40. Wculek, S.K. et al. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 20, 7-24 (2020).
41. Chan, C.W. & Housseau, F. The ‘kiss of death’ by dendritic cells to cancer cells. Cell Death Differ 15, 58-69 (2008).
42. Rolinski, J. & Hus, I. Breaking immunotolerance of tumors: a new perspective for dendritic cell therapy. J Immunotoxicol 11, 311-318 (2014).
43. Halpert, M.M. et al. Dendritic Cell-Secreted Cytotoxic T-Lymphocyte-Associated Protein-4 Regulates the T-cell Response by Downmodulating Bystander Surface B7. Stem Cells Dev 25, 774-787 (2016).
44. Pala, L. et al. Association of Anticancer Immune Checkpoint Inhibitors With Patient-Reported Outcomes Assessed in Randomized Clinical Trials: A Systematic Review and Meta-analysis. JAMA Netw Open 5, e2226252 (2022).
45. Brown, T.J., Mamtani, R. & Bange, E.M. Immunotherapy Adverse Effects. JAMA Oncol 7, 1908 (2021).
46. Barber, F.D. Adverse Events of Oncologic Immunotherapy and Their Management. Asia Pac J Oncol Nurs 6, 212-226 (2019).
READ THIS NEXT
Metastatic Melanoma: A Deep Dive into the Aggressive Form of Skin Cancer
Melanoma, a type of skin cancer, can be a frightening diagnosis. But within the spectrum of melanoma, metastatic melanoma stands out as part
Read MoreWhy don’t Checkpoint Inhibitors Work More Often?
With immunotherapy really taking off in the past decade, many cancer patients are rightfully interested in adding this potential modality to
Read MoreImmunotherapy vs. Chemotherapy Side Effects: Comparing the Objectives
On August 4th, we posted an overview of how chemotherapeutic side effects differ from those experienced during immunotherapy. Informative, b
Read More