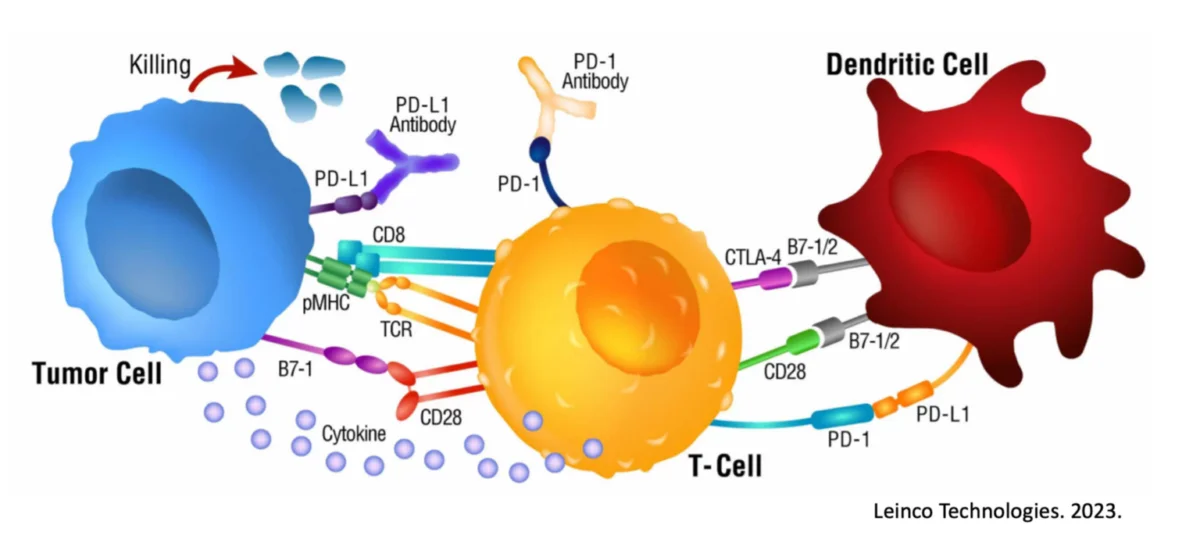
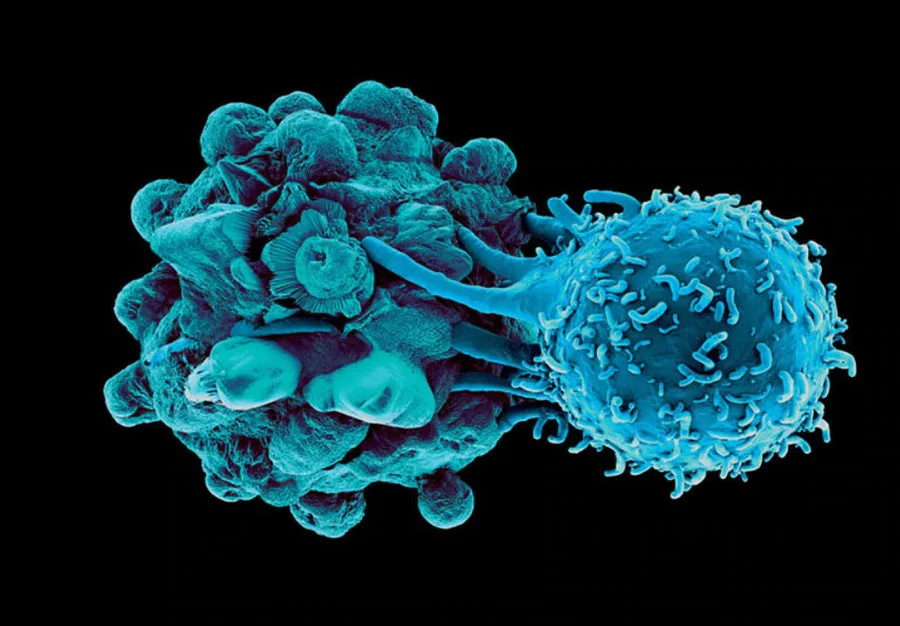
A New Alternative for Your Patients to Fight Cancer
Backed by decades of research and multiple ongoing FDA clinical trials, the Immunocine Dendritic Cell Treatment (IDCT) protocol is a less invasive, more precise way to treat multiple types of cancer.
Physician Quick Links
A Guide to Our Treatment
Read the science behind the Immunocine Dendritic Cell Treatment (IDCT) protocol, review the treatment timeline and meet our team.
The Immunocine Difference
Read the history behind Dendritic Cells, their attempts at use in cancer treatment and how our Double-Loading makes all the difference.
Fresh Tumoral Tissue Preservation
A guide to correctly preserving biopsied or resected tissue to prevent the degradation of genetic material and molecules for immunotherapy use.
Treatment Inclusion Criteria
Review the inclusion and exclusion criteria that all patients must meet in order to receive the Immunocine Dendritic Cell Treatment.

Treatment Timeline
Treatment is approximately 6 weeks long (non-inclusive), at which point a patient’s immune system is likely to be educated and engaged.
Our Only Priority is Treating Patients that Will Benefit from Our Treatment

Solid Tumors
While we are constantly expanding our capabilities, right now we only treat patients with solid tumors that can be biopsied by our medical staff. Blood malignancies, such as Leukemia, cannot be treated at this time.

Stable Immune System
For any immunotherapy to be successful, a patient needs to have a stable, functioning immune system. Patients with immune deficiencies and other conditions may not qualify for treatment until normal activity is restored.

Ability to Travel
Some medical conditions and other factors may prevent patients from traveling. Our treatment is administered exclusively at our facility in Cancún, Mexico. If you are unable to travel, you will not be able to receive our treatment.
Six-Week Treatment
Our treatment protocol requires three separate treatments, each two weeks apart. Patients can either choose to stay in Cancun for six weeks or visit twice for two weeks at a time.
Before considering our treatment as an option, please consider the inclusion criteria outlined. In order to be eligible, you must at least meet these requirements.

Partner With Us to Treat Your Patients
We partner with physicians regularly to provide the Immunocine Dendtritic Cell Treatment protocol to their late-stage cancer patients.

Paid Consultation
If your patient meets the minimum requirements and is interested in seeing how our treatment could work for their specific case, please submit their info on our submission form or call us directly at +1 (888) 575-2572.

Dedicated Support
Our team loves discussing our treatment with potential patients. We will schedule a call to learn about their diagnosis and answer initial questions. Prior to the call, we’ll share additional info for them to review with their physician.

Medical Evaluation
After confirming their eligibility, we will perform a complimentary medical evaluation. This includes a questionnaire and review of their medical records. Once complete, they can be approved for treatment.