Read the Peer-Reviewed Science Backing Our Treatment
Since its discovery in 2006 within the prestigious Texas Medical Center, our immunotherapy protocol has undergone continual research and clinical trials. This research has resulted in active clinical trials studying the treatment of Glioblastoma and Pancreatic Ductal Adenocarcinoma.
The following research is specific to Immunocine’s unique Dendritic Cell Treatment, which is patented and the first of its kind. These publications provide both scientific validation of our Immunotherapy as well as review the treatment’s pre-clinical and clinical successes.
The Immunocine Team recommends starting with the following four papers to develop a better mechanistic understanding of our treatment protocol and review both pre-clinical and clinical successes.
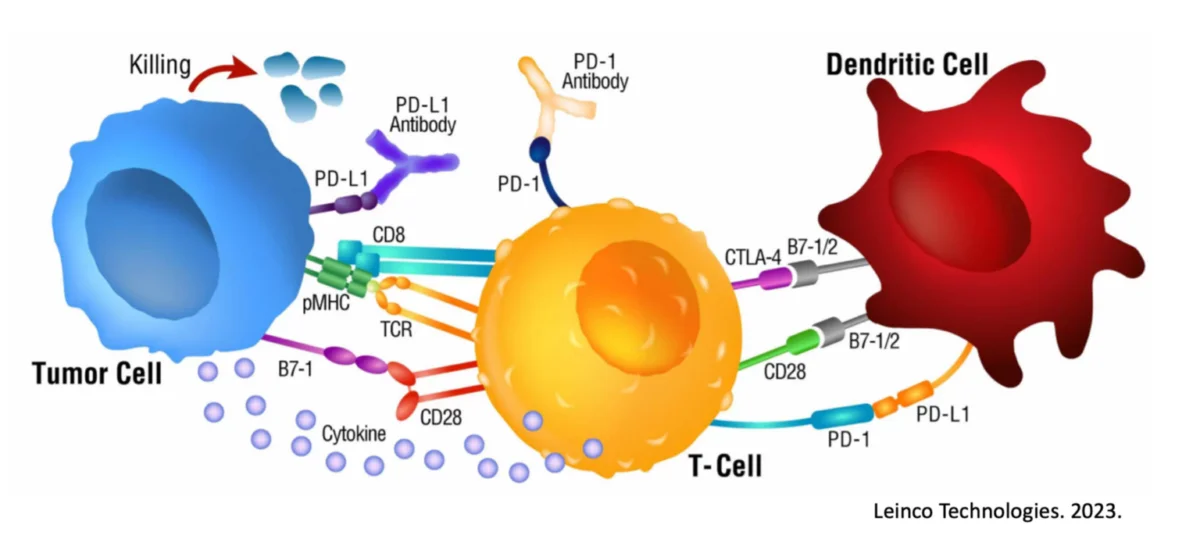
This phenomenon is demonstrated down to the MHC binding peptide level and is validated by a variety of measures including decreased CTLA-4 expression, increased CD83 levels, and increased IL-12 and Interferon-𝛾 secretion by the Dendritic Cells and responding T cells, respectively.
Taken together, it is apparent the Dendritic Cells possess a very detailed and important mechanism never previously identified and potentially critical to optimal immune responsiveness.
A change of even a couple of amino acids to eliminate the ‘100% overlap’ completely eliminates the response, demonstrating how fine-tuned and important this biological feat is. Unique to this ‘overlapped’ mechanism of Dendritic Cell loading is the downstream secretion of a powerful Th1 immune promoter, AIMp1 (a.k.a. p43) and the statistically significant upregulation of antigen-specific Killer T cells in circulation.
These results are repeatedly and eloquently shown through various experiments within this publication, and importantly it is also shown that other stimuli often used with Dendritic Cell or immunotherapy attempts (e.g., TLR agonists, bacterial components, viral components, interferons) were unable to replicate these responses, even when combined. The specific intracellular complex responsible for comparing MHC Class I and II bound sequences is also studied and demonstrated to be involved and critical.
Taken together, the findings published here are the detailed underpinnings of the mechanism driving the unique and powerful Dendritic Cell technology employed by Immunocine.
It is shown here that this type of immunotherapy (a.k.a. Dendritic Cell ‘overlapped’ loading with a target) can combine effectively with standard of care cancer treatments (e.g., chemotherapy), that the results are target-specific, and that the combination leads to significantly better treatment outcomes.
Importantly, it is thoroughly demonstrated that a truly unique population of memory T cells are propagated from this type (and only this type) of Dendritic Cell therapy, and that this greatly reduces the risk of relapse (as shown within the publication) and carries significant long-term protection capabilities.
Taken together, it has been shown that this powerful, specific, and special Th1 immune response has numerous unique outcome measures, is able to be translated from laboratory findings to preclinical successes in even very difficult to treat cancer models, and may in fact have strong utility in the future global treatment approach for cancer.
The Immunocine Team recommends starting with the following four papers to develop a better mechanistic understanding of our treatment protocol and review both pre-clinical and clinical successes.
A change of even a couple of amino acids to eliminate the ‘100% overlap’ completely eliminates the response, demonstrating how fine-tuned and important this biological feat is. Unique to this ‘overlapped’ mechanism of Dendritic Cell loading is the downstream secretion of a powerful Th1 immune promoter, AIMp1 (a.k.a. p43) and the statistically significant upregulation of antigen-specific Killer T cells in circulation.
These results are repeatedly and eloquently shown through various experiments within this publication, and importantly it is also shown that other stimuli often used with Dendritic Cell or immunotherapy attempts (e.g., TLR agonists, bacterial components, viral components, interferons) were unable to replicate these responses, even when combined. The specific intracellular complex responsible for comparing MHC Class I and II bound sequences is also studied and demonstrated to be involved and critical.
Taken together, the findings published here are the detailed underpinnings of the mechanism driving the unique and powerful Dendritic Cell technology employed by Immunocine.
It is shown here that this type of immunotherapy (a.k.a. Dendritic Cell ‘overlapped’ loading with a target) can combine effectively with standard of care cancer treatments (e.g., chemotherapy), that the results are target-specific, and that the combination leads to significantly better treatment outcomes.
Importantly, it is thoroughly demonstrated that a truly unique population of memory T cells are propagated from this type (and only this type) of Dendritic Cell therapy, and that this greatly reduces the risk of relapse (as shown within the publication) and carries significant long-term protection capabilities.
Taken together, it has been shown that this powerful, specific, and special Th1 immune response has numerous unique outcome measures, is able to be translated from laboratory findings to preclinical successes in even very difficult to treat cancer models, and may in fact have strong utility in the future global treatment approach for cancer.
Though not every dog was “cured,” 40% showed significant improvement including an example of long-term remission with no cancer identified at time of autopsy (death due to old age). These results provide preliminary evidence of the utility of this science in real cases, and suggests further study and use is extremely warranted.Code named APCEDEN in India, the treatment showed statistically significant improvement in survival, with limited adverse events, and ultimately received regulatory approval as a treatment within India for certain cancers. The lead author on this publication, as with many other publications we provide, is on the Immunocine team as this was a collaborative partnership.
Taken together, these results demonstrated conclusively the importance of Immunocine’s Th1-empowered Dendritic Cells in the ability to engender an anti-cancer immune response, and saw great first successes in efficacy with a reasonably and comparatively strong safety profile.
The following papers further elucidate and validate the mechanistic underpinnings of how the Immunocine’s unique approach to Dendritic Cell Treatments activates the required Th1 High immune response for effective cancer treatment.
In a variety of publications presented here, it is shown that Th1 ‘overlapped’ Dendritic Cells reduce their CTLA-4 expression and secretion and this leads to stronger anti-cancer immune responses. This publication was highlighted on the cover of the journal.
Cancer patients which spontaneously have higher levels of AIMp1 survive longer against cancer than patients deficient in AIMp1, and actually have a larger differential importance than Interferon-𝛾, as is shown within this publication.
Numerous publications herein clearly outlay that Dendritic Cells loaded with the Immunocine ‘overlapped’ protocol abundantly secrete and use AIMp1 to promote this uniquely powerful and specific Th1 anti-cancer response, while other Dendritic Cell or immunotherapy approaches fail to promote this effect.
This work dives into the natural genetic reprogramming of the T cells, what allows for their long-term memory, and how they are able to be so improved at killing target cells (e.g., cancer cells). Not only are these cells shown to be superior at cancer killing, but they continue to elucidate how the Immunocine Dendritic Cell treatment is unique, specific, and more powerful than other Dendritic Cell attempts which do not lead to the proliferation of these T cells.
Taken together, the entire Immunocine cellular pathway from education (Dendritic Cells) to effectiveness (unique Killer T cells) is being mapped out and understood to a level which allows for meticulous care in treatment and safety.
Start Your Personalized Treatment

1) Apply to See If You Qualify
If you meet the minimum requirements outlined above and are interested in seeing how our treatment could work for your specific case, please submit your info on our submission form or call us directly at +1 (888) 575-2572.

2) Schedule an Intro Call
Our team loves discussing our treatment with potential patients. We will schedule an intro call to learn about your diagnosis and answer any questions that you may have. Prior to the call, we will share additional info for you to review with your family and physician as you see fit.

3) Undergo Medical Evaluation
After confirming your eligibility, our Medical Team will perform a complimentary medical evaluation. This includes a questionnaire and review of your medical records. Once complete, you can be approved for treatment and we can build a personalized treatment program for you.