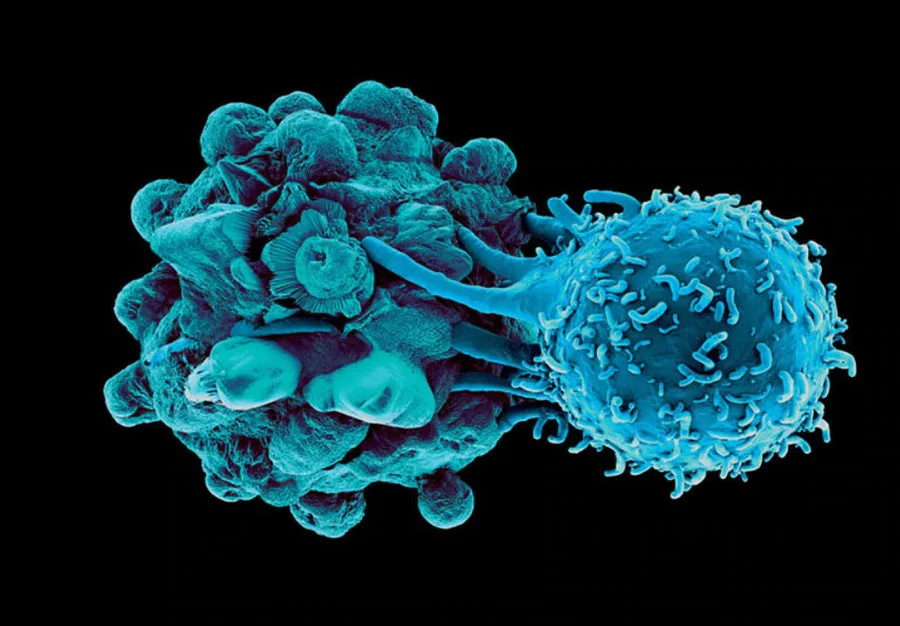
Dendritic Cells and Their Therapeutic Role in Prostate Cancer
In 2022, almost 270,000 American men will be diagnosed with prostate cancer, and about 35,000 prostate cancer patients will succumb to the disease.
Metastasized prostate cancer leaves only a 31% chance of a 5-year survival, and to date, prostate cancer is the 2nd leading cause of cancer-related death in men1. This disease continues to beg for newer and better treatment options.
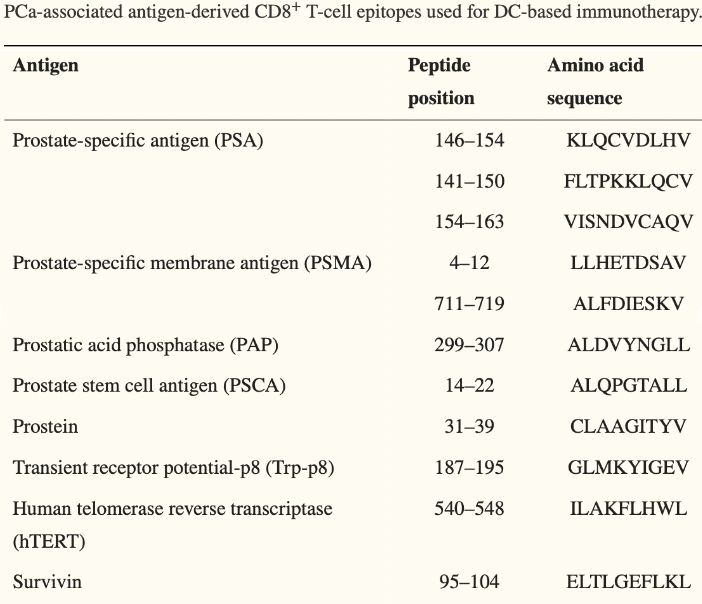
A number of therapies over the years have attempted to target “neoantigens (a.k.a. unique markers)” expressed on prostate tissue when it becomes cancerous2. And while it may make sense to target these markers, a couple of a major obstacles exist:
We, the doctors and scientists, have to know what those unique markers are so as to target them, and we don’t know many of them3.
Most of these therapies target a single antigen, which can easily be eliminated by the cancer to avoid immune targeting4, 5.
To overcome these obvious issues, therapies would ideally utilize machinery that is capable of targeting numerous (if not all) unique antigens expressed on prostate cancer tissue.
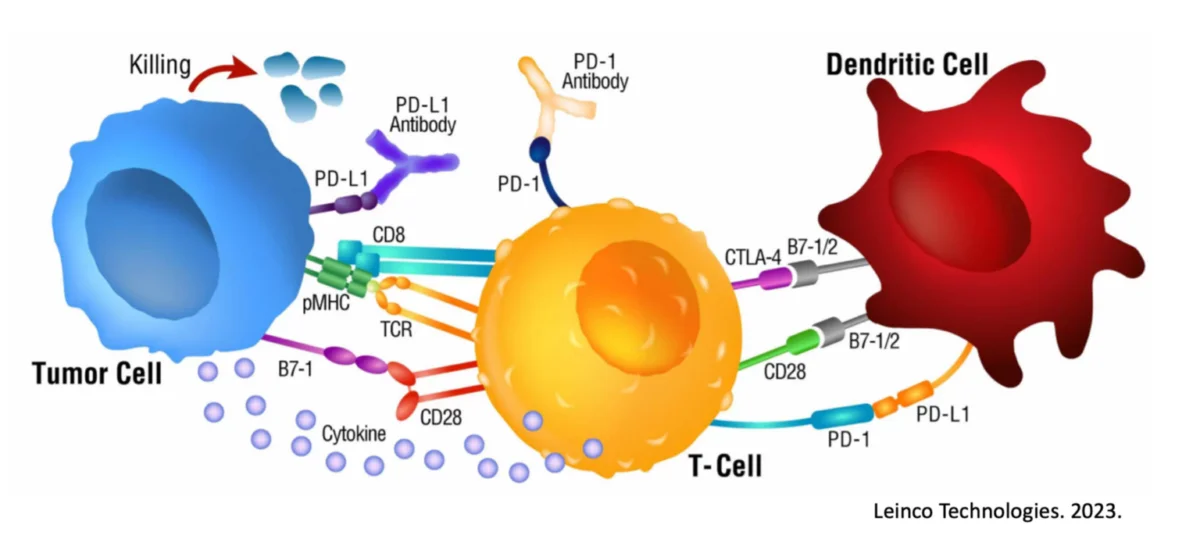
Dendritic Cells (DC) are the most efficient antigen-presenting cell (APC) in the body, supremely capable of orchestrating an immune response against an identified threat (for example, cancer)6. This requires 3 distinct DC-led features including:
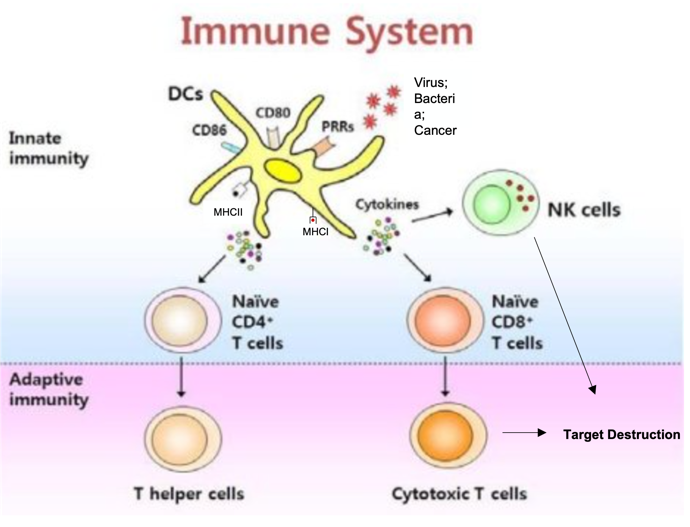
Learning of the threat and educating the rest of the immune system to the target
Activating the right kind of immune response needed to destroy such a target
Empowering the educated and empowered immune response to overcome obstacles that the cancer may attempt to utilize to escape destruction.
Awesomely, the DC is capable of absorbing and transmitting knowledge of ALL unique cancer markers expressed on the diseased prostate to the killer T cells, allowing them to have full understanding of the threat. Almost as if understanding how important DC are to fighting off cancer, prostate cancer imparticular has shown a unique ability in patients to restrict DC infiltration in the area7, 8, as well as inhibit activation of DC that manage to sample the tissue9, 10, 11. Given the extent which prostate cancer goes to deter and inactivate DC, it makes sense that the therapeutic use of DC to overcome prostate cancer would seem enticing.
Not surprisingly, a number of clinical efforts focused on the use of DC to treat cancer have been attempted. In 3 different trials, the administration of DCs pulsed with 3 distinct prostate peptides was well tolerated and resulted in the induction of immunological and clinical responses in patients12, 13, 14. Though much was left to be desired, safety along with immune inductions (to some degree) were demonstrated. A later trial utilizing DCs and 2 different peptides at the same time (PSA and PSCA) showed more significant survival advantages compared to previous attempts15. After that, a DC prostate cancer trial was conducted in which 3 different PSA peptides were used at the same time, which (aside from being safe) showed delayed tumor progression in 33% of the men, and a stable or reduced disease in 50%16. Though it is unnecessary to go through all iterations of studies over the years, it has become evident that DC plus a more expansive cancer antigenic library can lead to positive results in the case of prostate cancer situations.
Ultimately, these results helped to inform the FDA decision to officially approve a DC treatment for prostate cancer (called Provenge / Sipuleucel-T) in 201017. However, the technology behind how the DC was being used in this treatment was still in its infancy and far inferior to where we stand today. Within their own review of the trial results:
“The median time to disease progression was not statistically significant at 11.7 weeks in the vaccine group compared with 10.0 weeks in the placebo group. However, a statistically significant increase in median overall survival was observed (25.9 months in the vaccine group; 21.4 months in the placebo group).
For as amazing as the science and many of the results have been up to this point, this data leaves much to be desired18. Something was missing.
Bouncing off the “success” of Provenge, many cancer-treatment groups claim to offer a similar therapy to treat various cancers today. They like to highlight how the “revolutionary” Provenge treatment is FDA approved and therefore phenomenal, and they basically offer the same thing. The thing is, they do basically offer the same thing because the how they use their DC isn’t much different. Understanding that the how being used is suboptimal (to say the least), they may try and add some ‘razzle-dazzle’ by artificially stimulating with bacterial or viral toxins, but these don’t promote an anti-cancer response (and why would they?).

If the results shown with Provenge are your goal in an immunotherapy treatment for prostate cancer, then you have no shortage of options. If you desire better, and if the how is important to you, then Immunocine Dendritic Cells are now available to you. Exclusive and advanced, you owe it to yourself to ……
1. Sekhoacha, M. et al. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 27 (2022).
2. Gao, Y. et al. Identification of Neoantigens and Construction of Immune Subtypes in Prostate Adenocarcinoma. Front Genet 13, 886983 (2022).
3. Takamizawa, S. et al. Diagnostic value of tumor markers in identifying favorable or unfavorable subsets in patients with cancer of unknown primary: a retrospective study. BMC Cancer 22, 412 (2022).
4. Dhatchinamoorthy, K., Colbert, J.D. & Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front Immunol 12, 636568 (2021).
5. Boichard, A. & Kurzrock, R. Variable Mutation Expression in Human Cancers: A “Hide-and-Seek” Mechanism Linked to Differential MHC-I Presentation Dynamics. Mol Cancer Ther 21, 1219-1226 (2022).
6. Bol, K.F. et al. The clinical application of cancer immunotherapy based on naturally circulating dendritic cells. J Immunother Cancer 7, 109 (2019).
7. Troy, A., Davidson, P., Atkinson, C. & Hart, D. Phenotypic characterisation of the dendritic cell infiltrate in prostate cancer. J Urol 160, 214-219 (1998).
8. Aalamian, M. et al. Human prostate cancer regulates generation and maturation of monocyte-derived dendritic cells. Prostate 46, 68-75 (2001).
9. Aalamian-Matheis, M. et al. Inhibition of dendritic cell generation and function by serum from prostate cancer patients: correlation with serum-free PSA. Adv Exp Med Biol 601, 173-182 (2007).
10. Aalamian, M. et al. Inhibition of dendropoiesis by tumor derived and purified prostate specific antigen. J Urol 170, 2026-2030 (2003).
11. Bai, W.K., Zhang, W. & Hu, B. Vascular endothelial growth factor suppresses dendritic cells function of human prostate cancer. Onco Targets Ther 11, 1267-1274 (2018).
12. Murphy, G., Tjoa, B., Ragde, H., Kenny, G. & Boynton, A. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate 29, 371-380 (1996).
13. Tjoa, B.A. et al. Follow-up evaluation of prostate cancer patients infused with autologous dendritic cells pulsed with PSMA peptides. Prostate 32, 272-278 (1997).
14. Jahnisch, H. et al. Dendritic cell-based immunotherapy for prostate cancer. Clin Dev Immunol 2010, 517493 (2010).
15. Thomas-Kaskel, A.K. et al. Vaccination of advanced prostate cancer patients with PSCA and PSA peptide-loaded dendritic cells induces DTH responses that correlate with superior overall survival. Int J Cancer 119, 2428-2434 (2006).
16. Hildenbrand, B. et al. Immunotherapy of patients with hormone-refractory prostate carcinoma pre-treated with interferon-gamma and vaccinated with autologous PSA-peptide loaded dendritic cells–a pilot study. Prostate 67, 500-508 (2007).
17. Drugs.com. Provenge FDA Approval History. 2010 [cited]Available from: https://www.drugs.com/history/provenge.html
18. LLC, D.P. Provenge. 2021. Available from: https://provenge.com/hcp/prolonged-survival
READ THIS NEXT
Overcoming a Rare & Aggressive Kidney Cancer with Immunocine: Justin’s Story
In the prime of his life, Justin was living the life many dream of — raising three young children with his wife and staying in peak physic
Read MoreOn Air with Immunocine: Matt Halpert Joins Haylie Pomroy to Discuss the Future of Cancer Treatment
Listen to this Episode on Apple Podcasts Listen to this Episode on Spotify In this episode of the Hope and Help for Fatigue and Chronic Illn
Read MoreLife After Cancer: Kevin’s Journey with Stage 2C Melanoma Cancer
Kevin, an avid dirt biker and owner of a excavation company operating heavy machinery, faced his toughest challenge not in the wild but in a
Read More