Inflammation in Cancer
In today’s world, chronic inflammation is an ever-present challenge linked to serious diseases, notably cancer. However, the paradox arises when we consider immunotherapy—a treatment harnessing inflammation to combat cancer. The key to understanding this complex interplay lies in precise “targeting” of the immune system’s arsenal. While chronic inflammation can fuel cancer, abandoning it entirely cedes biology to the tumor. Instead, we must learn to wield inflammation strategically. Tumors exploit non-specific inflammation through mechanisms like HrH1 receptors and immune cell recruitment, but promising strategies such as HrH1 antagonists and repurposed drugs are emerging. Furthermore, a more targeted approach involving Dendritic Cells holds potential as these immune generals orchestrate specific responses against cancer cells. In essence, the relationship between inflammation and cancer is nuanced, emphasizing the need to differentiate between indiscriminate and targeted inflammation to effectively combat this formidable adversary. The key takeaway is to optimize our natural defenses, fighting cancer’s attempts to hijack our biology with superior strategies, and once harnessed, the true battle against cancer can commence.
Context is Everything
In general, it has been accepted that we are a people that deal with an unprecedented amount of chronic inflammation1. Whether it be from prolonged sitting at a desk2, eating a western diet3, or inconsistent sleep4, it would seem difficult to avoid being in a state of at least some ongoing inflammation. It is also generally considered that chronic inflammation can lead to serious diseases, including cancer5, which often seem to evolve out of a pit of ‘inflamed swamp grounds’ resident to our bodies. How then do we reconcile immunotherapy for cancer, which by nature elicits an inflammatory response designed to infiltrate tumors? Understanding these differences may be a useful tool if / when dealing with cancer.
A key term here will be “targeting,” as that is ultimately what makes the difference. The immune system is eloquently capable of defeating threats, including cancers, and has different weapons to call upon based on the situation6. There are times when a very specified, narrow attack is needed in which, for example, killer T cells will select cells that need to be eliminated in order to avoid further trouble7. There are other times in which the immune system decides to make the entire area inhospitable for an invading pathogen, and widespread destruction is permitted and promoted8. Cancer specifically utilizes the latter, hijacking it for its own nefarious desires to break out and spread throughout the body9. To now completely avoid inflammatory processes is to cede biology to the cancer. Instead of giving up one of your best weapons, learn to better focus it and ‘outsmart’ the insidious tumor.
Tumors attempt to utilize non-specific inflammation to cause chaos and destruction to the surrounding environment to aid its growth and spread. One such example is promoting signaling through the HrH1 receptor, also known as Histamine receptor H1, a receptor commonly stimulated by histamine10, 11. Histamine is a chemical that is produced by cells in your body, most commonly mast cells. It is usually released when your body comes into contact with something that it sees as a foreign invader that needs immediate expulsion, such as parasites, or mistargeted against things like pollen, dust, or pet dander12. Histamine causes the symptoms of an allergic reaction, such as sneezing, itching, and hives, or more severe events such as anaphylactic shock13. Histamine is also involved in tumor growth and progression11, 14. Tumor cells can also produce histamine, which can promote tumor growth by stimulating angiogenesis (the growth of new blood vessels) and by increasing the production of growth factors15. Histamine can also make tumors more resistant to chemotherapy, radiation therapy, and predominately immunotherapy16, 17. Because of this potential pro-tumorigenesis role, HrH1 antagonists such as Zyrtec and Claritin may be useful in delaying tumor progression16, 18, 19, 20. And indeed, these are being tested now in a variety of cancer clinical trials.
Another way tumors may hijack a widespread inflammatory response for its aid is to recruit neutrophils, a ‘first-responder’ to bacteria and immediate pathogenic threats once they break the physical barriers of your body21, 22. Neutrophils can fight a variety of ways, including the release of Reactive Oxygen Species (ROS)23 and Neutrophil Extracellular Traps (NETs) which function like spider webs to trap threats in a toxic concoction that even includes the neutrophils own DNA24. These NETS have also been found to assist tumor growth, and as such, treatments against neutrophils and NETs have demonstrated an ability to combat cancer progression25, 26. Macrophage, another quick responder, is also commonly used by tumors to further exacerbate this inflammatory event27. Recently, repurposing Disulfiram (commonly as known Antabuse) has moved forward as a drug of focus based on its ability to reduce both neutrophil accumulation and macrophage activity within tumor microenvironments. Research has demonstrated that it is a potent inhibitor of signaling pathways in M2 (e.g., tumor-promoting) macrophages and the blocking of NET release by neutrophils28, 29, 30. Though Disulfiram may possess a side effect profile that need to be carefully analyzed in the context of the entire patient, it may be another “easy add” for patients with immunosuppressive cancers.
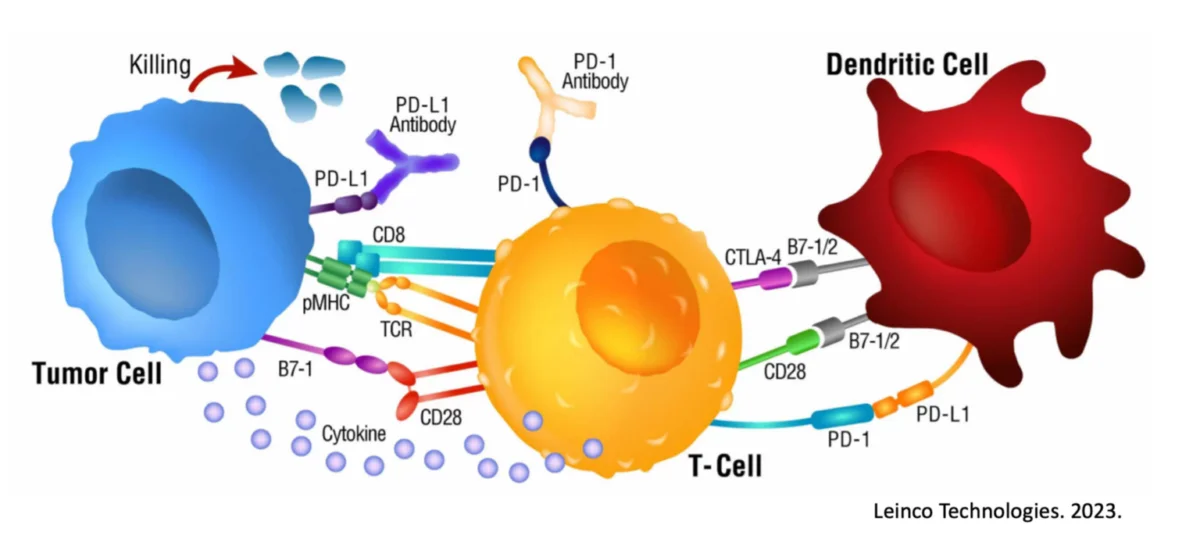
Working through the adaptive side of the immune system allows for targeted, highly-specific immune responses which can focus inflammatory processes against individual cells of a cancer. In this way, “inflammation” is a positive as part of a treatment plan and needs to not only be incorporated, but possibly prioritized. CAR-T cells are one such mechanism to attempt to engender an antigen-specific immune response against particular tumor cells31, 32, but does require genetic manipulations in the lab and also runs into a number of obstacles, including antigenic shedding (e.g., a process by which the tumor eliminates the 1 or 2 antigens the CAR-T cells may be targeting, rendering the tumor invisible)33, 34, 35.
A more physiological approach would be to work from the ‘top-down’ and use Dendritic Cells, which function as the ‘generals’ of the immune system ‘army’ and are capable of learning and incorporating the entire blueprints of what a cancer is and could be36, 37. In this way, they can communicate effectively to the killer T cells (e.g., the ‘foot soldiers’) all needed information to incite a robust and targeted immune response against the cancer cells. However, Dendritic Cells have only recently started to overcome their previous reputation of an incapable therapeutic (due to years and years of clinical failures) based on the important discovery of ‘double-loading’ their antigen pathways for full immune activation36, 38, 39, 40, 41. This discovery and usage in the clinic have changed the way we view Dendritic Cells and their potential and should once again elevate (correct) Dendritic Cell therapeutics to the front lines.
Taken all together, the concept of “inflammation” is clearly not straight forward and cannot be simply lumped into a “helpful” or “hurtful” category. Context is everything and targeted vs. non-descript can lead to very different outcomes in the case of cancer. A general suggestion at this time regarding “how one should view inflammation during cancer” is to attempt to minimize non-specific, hijacked inflammatory players (e.g., histamine, neutrophils, etc.) which can be partly accomplished by lifestyle changes, and optimize cancer-targeted inflammatory processes the body normally utilizes to fight such a threat (e.g., double-loaded Dendritic Cells, antigen-specific immunotherapy). Do not give up your cellular abilities (and your homefield advantage) just because cancer has attempted to steal it from you. Fight this dysregulated biology with superior and empowered biology. Once harnessed, the fight can truly begin.
- Pahwa, R., Goyal, A. & Jialal, I. Chronic Inflammation. StatPearls: Treasure Island (FL), 2023.
- Cabanas-Sanchez, V., Guallar-Castillon, P., Higueras-Fresnillo, S., Garcia-Esquinas, E., Rodriguez-Artalejo, F. & Martinez-Gomez, D. Physical Activity, Sitting Time, and Mortality From Inflammatory Diseases in Older Adults. Front Physiol 9, 898 (2018).
- Clemente-Suarez, V.J., Beltran-Velasco, A.I., Redondo-Florez, L., Martin-Rodriguez, A. & Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 15 (2023).
- Dzierzewski, J.M., Donovan, E.K., Kay, D.B., Sannes, T.S. & Bradbrook, K.E. Sleep Inconsistency and Markers of Inflammation. Front Neurol 11, 1042 (2020).
- Singh, N., Baby, D., Rajguru, J.P., Patil, P.B., Thakkannavar, S.S. & Pujari, V.B. Inflammation and cancer. Ann Afr Med 18, 121-126 (2019).
- Margraf, A. & Perretti, M. Immune Cell Plasticity in Inflammation: Insights into Description and Regulation of Immune Cell Phenotypes. Cells 11 (2022).
- Spetz, J., Presser, A.G. & Sarosiek, K.A. T Cells and Regulated Cell Death: Kill or Be Killed. Int Rev Cell Mol Biol 342, 27-71 (2019).
- Chen, L. et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9, 7204-7218 (2018).
- Liu, J., Lin, P.C. & Zhou, B.P. Inflammation fuels tumor progress and metastasis. Curr Pharm Des 21, 3032-3040 (2015).
- Mizuguchi, H. et al. Signaling Pathway of Histamine H(1) Receptor-Mediated Histamine H(1) Receptor Gene Upregulation Induced by Histamine in U-373 MG Cells. Curr Issues Mol Biol 43, 1243-1254 (2021).
- Sarasola, M.P., Taquez Delgado, M.A., Nicoud, M.B. & Medina, V.A. Histamine in cancer immunology and immunotherapy. Current status and new perspectives. Pharmacol Res Perspect 9, e00778 (2021).
- Thangam, E.B. et al. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front Immunol 9, 1873 (2018).
- Lieberman, P. The basics of histamine biology. Ann Allergy Asthma Immunol 106, S2-5 (2011).
- Zhao, J. et al. Upregulation of histamine receptor H1 promotes tumor progression and contributes to poor prognosis in hepatocellular carcinoma. Oncogene 39, 1724-1738 (2020).
- Chen, J. et al. Glioblastoma stem cell-specific histamine secretion drives pro-angiogenic tumor microenvironment remodeling. Cell Stem Cell 29, 1531-1546 e1537 (2022).
- Li, H. et al. The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1. Cancer Cell 40, 36-52 e39 (2022).
- Somasundaram, R. et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. Nat Commun 12, 346 (2021).
- Bai, Z., Ai, J. & Wei, X. Histamine and histamine receptor H1 (HRH1) axis: new target for enhancing immunotherapy response. Mol Biomed 3, 11 (2022).
- Fernandez-Nogueira, P. et al. Histamine receptor 1 inhibition enhances antitumor therapeutic responses through extracellular signal-regulated kinase (ERK) activation in breast cancer. Cancer Lett 424, 70-83 (2018).
- Fritz, I., Wagner, P. & Olsson, H. Improved survival in several cancers with use of H(1)-antihistamines desloratadine and loratadine. Transl Oncol 14, 101029 (2021).
- Yan, M. et al. Roles of tumor-associated neutrophils in tumor metastasis and its clinical applications. Front Cell Dev Biol 10, 938289 (2022).
- Malech, H.L., DeLeo, F.R. & Quinn, M.T. The Role of Neutrophils in the Immune System: An Overview. Methods Mol Biol 2087, 3-10 (2020).
- Nguyen, G.T., Green, E.R. & Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front Cell Infect Microbiol 7, 373 (2017).
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 18, 134-147 (2018).
- Li, J., Chen, J., Sun, J. & Li, K. The Formation of NETs and Their Mechanism of Promoting Tumor Metastasis. J Oncol 2023, 7022337 (2023).
- Yang, S. et al. Neutrophil extracellular traps promote angiogenesis in gastric cancer. Cell Commun Signal 21, 176 (2023).
- Qian, B.Z. & Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39-51 (2010).
- Terashima, Y. et al. Targeting FROUNT with disulfiram suppresses macrophage accumulation and its tumor-promoting properties. Nat Commun 11, 609 (2020).
- Adrover, J.M. et al. Disulfiram inhibits neutrophil extracellular trap formation and protects rodents from acute lung injury and SARS-CoV-2 infection. JCI Insight 7 (2022).
- Meraz-Torres, F., Ploger, S., Garbe, C., Niessner, H. & Sinnberg, T. Disulfiram as a Therapeutic Agent for Metastatic Malignant Melanoma-Old Myth or New Logos? Cancers (Basel) 12 (2020).
- Caraffa, A., Gallenga, C.E., Kritas, S.K., Ronconi, G., Di Emidio, P. & Conti, P. CAR-T cell therapy causes inflammation by IL-1 which activates inflammatory cytokine mast cells: anti-inflammatory role of IL-
- J Biol Regul Homeost Agents 33, 1981-1985 (2019).
- Benmebarek, M.R., Karches, C.H., Cadilha, B.L., Lesch, S., Endres, S. & Kobold, S. Killing Mechanisms of Chimeric Antigen Receptor (CAR) T Cells. Int J Mol Sci 20 (2019).
- Sterner, R.C. & Sterner, R.M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 11, 69 (2021).
- Strati, P. & Neelapu, S.S. CAR-T failure: beyond antigen loss and T cells. Blood 137, 2567-2568 (2021).
- Majzner, R.G. & Mackall, C.L. Tumor Antigen Escape from CAR T-cell Therapy. Cancer Discov 8, 1219-1226 (2018).
- Wculek, S.K., Cueto, F.J., Mujal, A.M., Melero, I., Krummel, M.F. & Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 20, 7-24 (2020).
- Marciscano, A.E. & Anandasabapathy, N. The role of dendritic cells in cancer and anti-tumor immunity. Semin Immunol 52, 101481 (2021).
- Decker, W.K. et al. Th-1 polarization is regulated by dendritic-cell comparison of MHC class I and class II antigens. Blood 113, 4213-4223 (2009).
- Halpert, M.M. et al. Dendritic Cell-Secreted Cytotoxic T-Lymphocyte-Associated Protein-4 Regulates the T-cell Response by Downmodulating Bystander Surface B7. Stem Cells Dev 25, 774-787 (2016).
- Halpert, M.M. et al. MHC class I and II peptide homology regulates the cellular immune response. FASEB J 34, 8082-8101 (2020).
- Konduri, V. et al. Dendritic cell vaccination plus low-dose doxorubicin for the treatment of spontaneous canine hemangiosarcoma. Cancer Gene Ther 26, 282-291 (2019).
READ THIS NEXT
Overcoming a Rare & Aggressive Kidney Cancer with Immunocine: Justin’s Story
In the prime of his life, Justin was living the life many dream of — raising three young children with his wife and staying in peak physic
Read MoreOn Air with Immunocine: Matt Halpert Joins Haylie Pomroy to Discuss the Future of Cancer Treatment
Listen to this Episode on Apple Podcasts Listen to this Episode on Spotify In this episode of the Hope and Help for Fatigue and Chronic Illn
Read MoreLife After Cancer: Kevin’s Journey with Stage 2C Melanoma Cancer
Kevin, an avid dirt biker and owner of a excavation company operating heavy machinery, faced his toughest challenge not in the wild but in a
Read More