A Revolutionary Approach to Cancer Treatments
Immunocine’s Dendritic Cell Treatment represents a huge step forward in Immunotherapy and utilizing Dendritic Cells to fight cancer.
In fact, the following data demonstrates how much more powerful Immunocine’s Treatment is than other Dendritic Cell Treatments.
However, before understanding what makes Immunocine’s Treatment so powerful, we believe it is important to understand why Dendritic Cells hold so much potential in cancer treatments.
The Promise of Dendritic Cell Treatments
A Dendritic Cell is a special type of immune cell that is found throughout the body’s tissues. It has been long believed Dendritic Cells boost immune responses by showing antigens on their surface to other cells of the immune system. However, new research suggests that Dendritic Cells can lead the attack, not just support an immune response.
More simply, the Dendritic Cells act as the “general” of the immune system in directing the attacks against viruses, foreign bacteria, and mutated cells. This natural and existing role within the immune system, gives Dendritic Cells significant advantages as part of a cancer treatment.
Dendritic Cells already play a critical role in anti-tumor activity. Dendritic Cells specifically activate CD8+ T cells, which are widely regarded as the major anti-tumor and antiviral mechanism of the body.
Dendritic Cells are natural. Dendritic Cell Treatments do not introduce any medication or genetically modified cells into the body. This results in a significantly reduced risk of side effects.
Dendritic Cells can initiate immunological memory. Once the immune system is aware of a threat, it becomes much better at identifying and handling a repeat encounter of that threat.
Why Are DC Treatments not the standard of care?
Unfortunately, every prior attempt at Dendritic Cell Treatments failed to properly activate a patient’s Dendritic Cells. Starting in the 1990’s, Researchers and companies alike tried a variety of approaches to introduce the patient’s cancerous cells to their Dendritic Cells.
Dendritic Cells mixed with Cancerous Cell Parts (“Lysate”). Hoping that simply placing cancer antigens next to the Dendritic Cells would be enough, drugs like Sipuleucel-T were some of the first FDA-approved Dendritic Cell Treatments. To further bolster the immune response, Provenge also adds immune-boosting proteins to the mix.
DC mixed with Cancerous Cells + Bacteria (“Lysate 2.0”). Believing the key to activating Dendritic Cells was actually mimicking a bacterial infection, multiple treatment attempts have tried combining cancerous cells with bacterial components such as BCG (which naturally leads to tuberous sclerosis). Others have tried to use toxins collected from dead bacteria, often called “Coley Toxins”, based on discoveries in 1893. While this can provide some benefit, there is a reason this approach is not widely used 129 years later!
DC injected with Cancer mRNA (“mRNA”). Attempting to force an interaction between the cancer and Dendritic Cells, treatments such as Rocapuldencel-T inject the cancer mRNA inside of the Dendritic Cell. This approach has also fallen short with Rocapuldencel-T prematurely ending its most recent clinical trial for Renal Cell Carcinoma.
Immunocine’s Treatment Simulates a Viral Infection
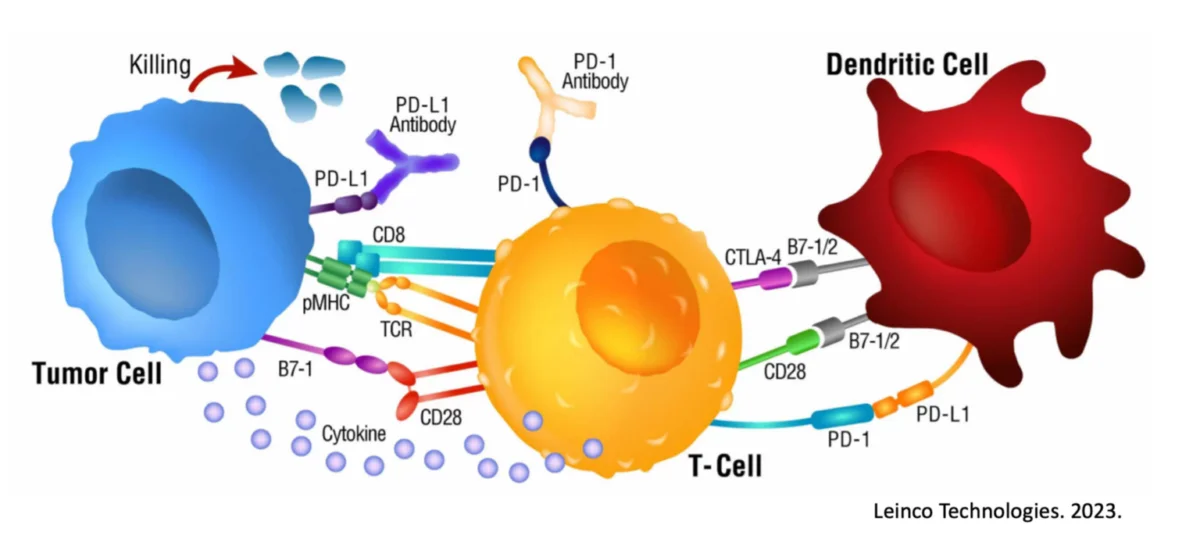
Despite each of these approaches having unique variations, each prior Dendritic Cell Treatment is identical in that the cancerous cells are presented either internally or externally, but not both at the same time. Therefore, these cancer treatments only activate one of the MHC Class pathways in Dendritic Cells.
While you do not need to know what an MHC Class pathway is, just know that activating only one pathway will generate either a bacterial immune response or simply no immune response at all. Given that tumors are not foreign bacteria, it is not surprising why these treatments have failed.
Instead, the Immunocine Treatment is the first and only treatment that simultaneously activates both MHC Class pathways in Dendritic Cells. This dual activation replicates a viral infection, which is exactly what is needed to destroy cancer cells.
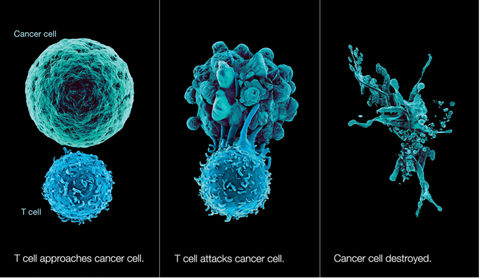
In a viral infection, the infected cells stop their normal functions and instead start producing more and more copies of the virus. Therefore, the body’s first priority in fighting a virus is aggressively destroying any infected cells to stop any additional viral production. This response has to be very fast and powerful to keep up with the viral growth.
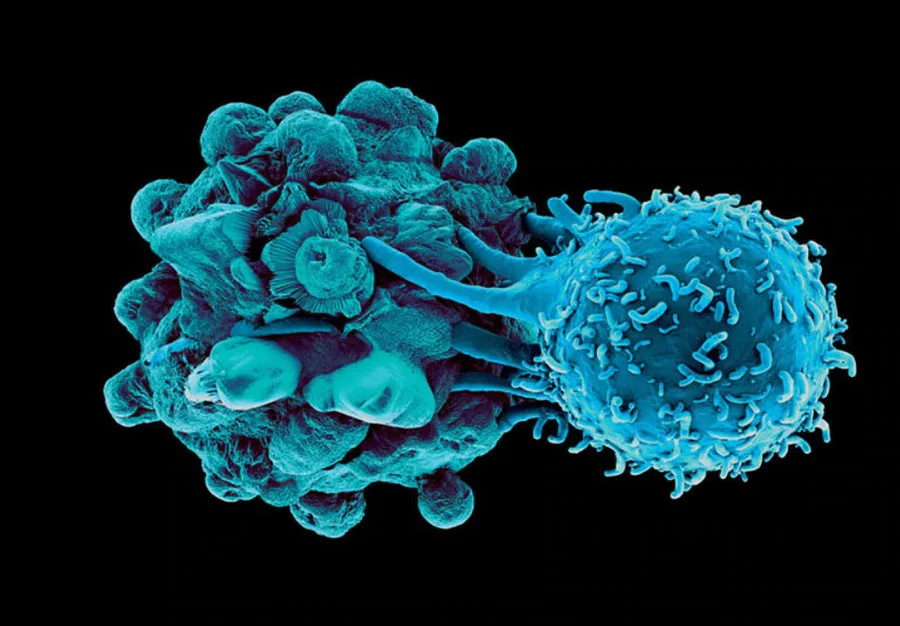
No different than a viral infection, the Immunocine Treatment’s causes the Dendritic Cells to activate the powerful CD8+ T cells to destroy all “infected cells”. However, instead of infected cells, the patient’s immune system is now aggressively looking to destroy cancer cells and stop their spread!
The Power of Immunocine’s Approach
While Immunocine’s Treatment is proud to provide the only treatment that simulates a viral infection, nothing matters more than the ultimate performance of this unique approach. To determine if the Immunocine Dendritic Cell Treatment provided any benefit over the competing treatments, the following experiment was conducted.
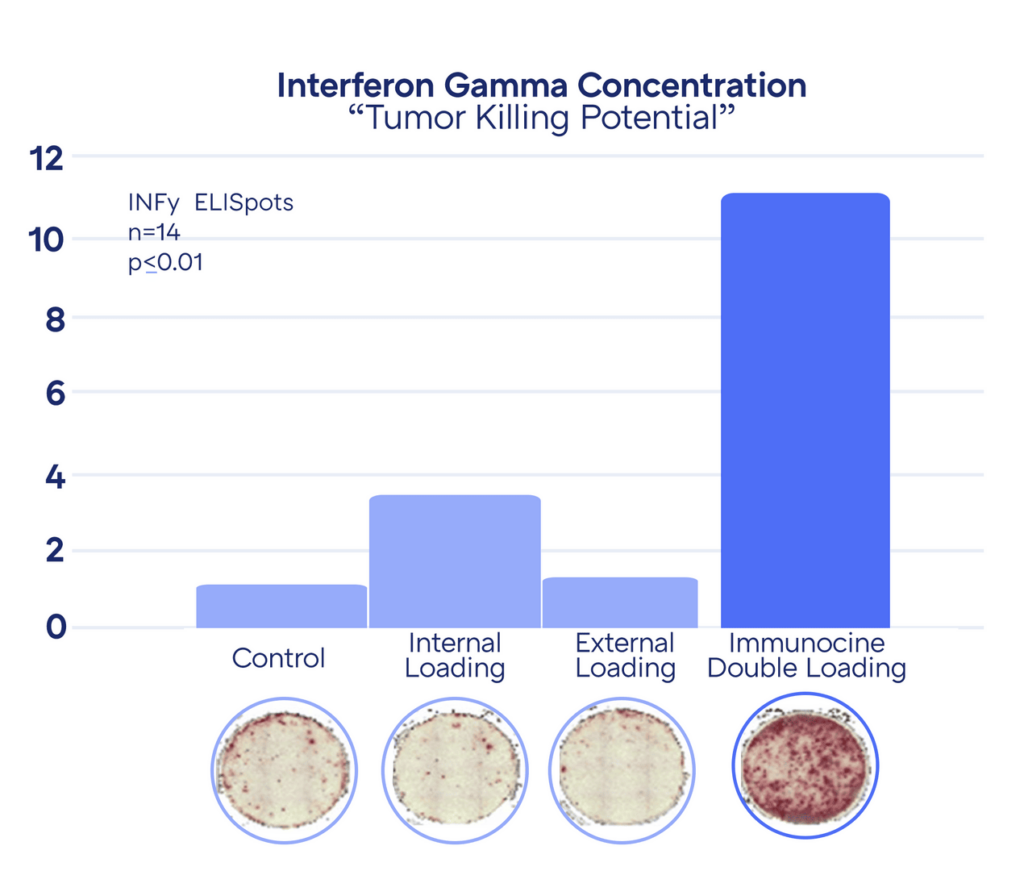
In the Dendritic Cell activation, the powerful CD8+ T cells release a compound called Interferon Gamma, which further activates surrounding T cells to start seeking out infected or mutated cells that need to be destroyed. Therefore, the “anti-tumor potential” of each Dendritic Cell treatment can be estimated by the amount of Interferon Gamma subsequently released.
The higher concentration of Interferon Gamma correlates to more “red spots” in the Petri dish. As can be seen above, the anti-tumor activity of Immunocine’s treatment is significantly stronger than the existing Dendritic Cell Treatments. This has been replicated countless times since the initial discovery in 2006.
Proven, Real World Results
After the initial discovery of Immunocine’s unique approach to using Dendritic Cells for cancer treatment, the scientific team spent the next 15 years researching and validating this technique at the Texas Medical Center, one of the world’s leading cancer research hubs. This has resulted in over 17 publications in peer reviewed journals, several clinical studies, and now the treatment protocol is being studied in multiple FDA Clinical Trials.
As part of its validation, the Immunocine Treatment was clinically analyzed in the treatment of the first 53 refractory cancer patients who were no longer responding to standard of cancer treatments. This was a “basket-catch trial” and included patients with Ovary, Colon, Neck, Lung, Prostate Breast, Sarcoma, Cervical, Pancreatic, Kidney, and Melanoma cancers.
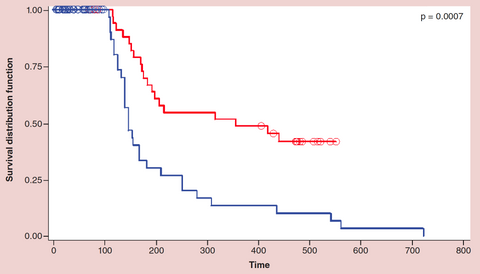
This chart represents the Long Term Survival of the patients in the clinical trial. The patients receiving Immunocine’s Treatment are represented with the Red Line, the control group is represented by the Blue Line.
Where the control group had a median survival of 157 days, the late-stage cancer patients receiving Immunocine’s Treatment had a median survival of 356 days. Not only did the average survival rate increase 2.26x, but the study revealed two additional important discoveries:
This trial included 13 different types of cancer, with each cancer showing distinct improvement over the control.
Despite being labeled as “incurable cancer patients”, 30% of the patients receiving Immunocine’s Treatment achieved long-term, indefinite survival. As these patients achieved long-term survival, they were ultimately removed from the study. If they were instead kept in the study, the average survival would have most likely increased dramatically over time.
Why do more people not know about this?
Cancer research takes an incredibly long time to complete and fortunately, a lot of progress has been made. Located within the Texas Medical Center, the founding researchers of this treatment protocol are now underway with multiple FDA Clinical Trials focused on Glioblastoma and Pancreatic Cancer, two of the most deadly and aggressive cancers.
READ THIS NEXT
On Air with Immunocine: Matt Halpert Joins Haylie Pomroy to Discuss the Future of Cancer Treatment
Listen to this Episode on Apple Podcasts Listen to this Episode on Spotify In this episode of the Hope and Help for Fatigue and Chronic Illn
Read MoreLife After Cancer: Kevin’s Journey with Stage 2C Melanoma Cancer
Kevin, an avid dirt biker and owner of a excavation company operating heavy machinery, faced his toughest challenge not in the wild but in a
Read MoreTeam Spotlight: Dr. Francisco Zago, MD
Dr. Francisco Zago, MD, joined Immunocine in 2023 and is now the Patient Care Director, bringing a wealth of experience in Primary Care. Wit
Read More