The Misconception of Checkpoint Inhibitors
As the earliest form of commercialized Immunotherapy, Checkpoint Inhibitors laid the foundation for Immunotherapy as a viable cancer treatment. Further, Checkpoint Inhibitors are able to be produced in bulk and are relatively safe especially when compared to Chemotherapy.
This initial success and their ease of use have made Checkpoint Inhibitors the most popular form of Immunotherapy. In fact, a specific Checkpoint Inhibitor, Keytruda, is the most widely used cancer drug in the US. Overall, this popularity has been great for helping patients fight cancer and raising awareness for the potential of Immunotherapy.
However, this popularity has resulted in many cancer patients and even some oncologists believing that Checkpoint Inhibitors represent all of Immunotherapy. As Checkpoint Inhibitors are often not curative for advanced or aggressive cancers, this misconception can leave many patients believing they have run out of treatment options and that “Immunotherapy” will not work for them.
To help separate truth from fiction, the Immunocine Team has put together a brief overview of Checkpoint Inhibitors and their role in fighting cancer.
Origin of Checkpoint Inhibitors
In the 1990’s, Dr. Jim Allison and colleagues discovered that the body uses numerous proteins to subdue the immune response, such as CTLA-4 and PD-1. Scientists believe we have this ability to prevent autoimmunity or excessive inflammation under natural circumstances.
However, these same “quieting” signals can also be hijacked and ultimately used to undermine the Immune System’s ability to destroy cancer cells.
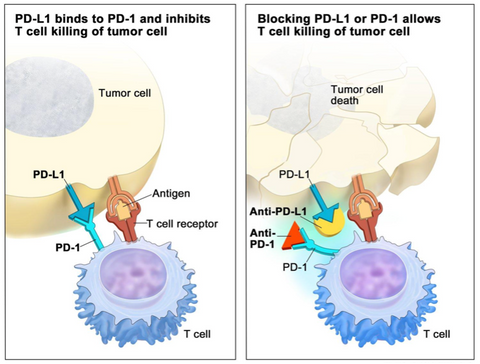
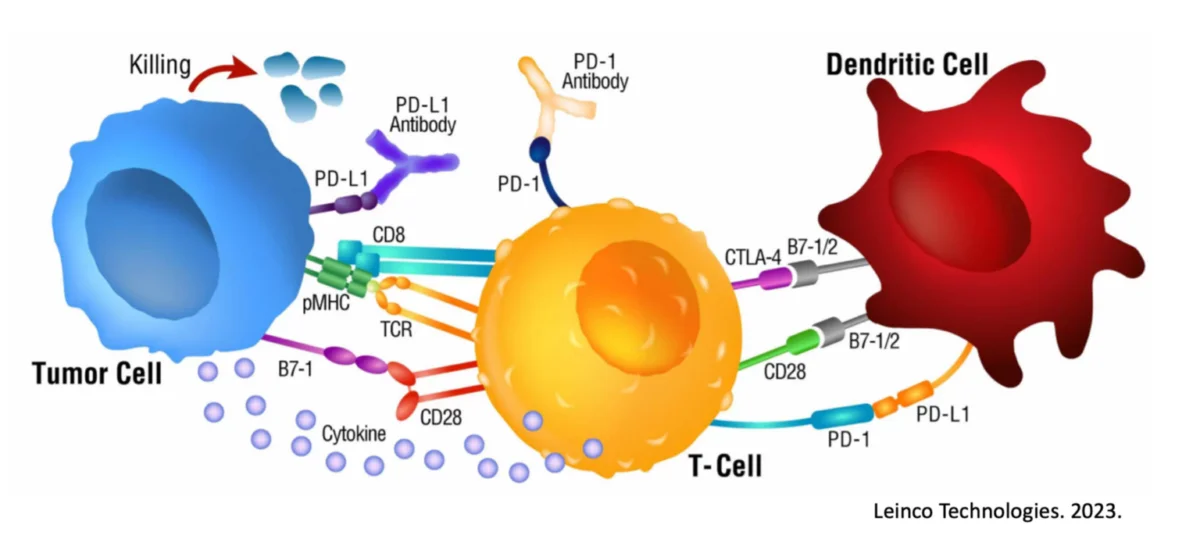
When an immune cell detects either of these regulatory proteins on a cell’s surface, the immune cell will naturally ignore the investigated cell. Research has now confirmed that cancer cells can mirror this behavior and also turn off a response. In short, cancer cells are even more sinister in that they actively hide from the Immune System
Using Checkpoint Inhibitors for Treatment
To prevent cancer cells from hiding, scientists have created a category of drugs called “Checkpoint Inhibitors”. Checkpoint Inhibitors block the cancer’s ability to “turn off” the immune cells via these regulatory receptors (such as CTLA-4 and PD-1). Now no longer able to hide, the immune cells have an easier time identifying and destroying the cancer cells.
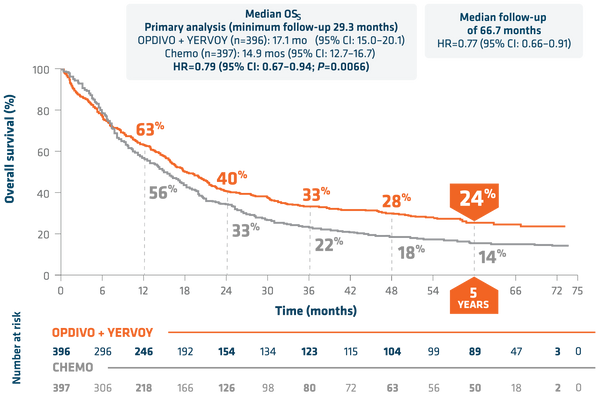
In 2011, the FDA approved Yervoy (Ipilimumab), which was the first Checkpoint Inhibitor to reach commercialization. Yervoy actively blocks CTLA-4 and is used for the treatment of metastatic melanoma as well as other cancers that may be attempting to utilize this pathway. For metastatic Non-Small Cell Lung Cancer, Yervoy with Opdivo improved 5 Year Overall Survival to 24% from 14% when compared to patients receiving Chemotherapy.
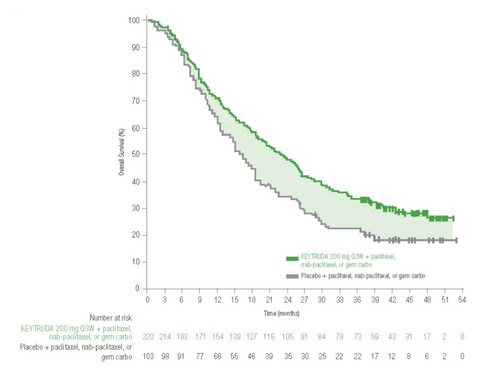
In 2017, the FDA expanded treatment indications for Keytruda (Pembrolizumab), which blocks PD-1, to include any metastatic or non-removable solid tumor with certain characteristics. Keytruda is now believed to be the most widely used cancer drug with over $17B in annual sales. For advanced Triple Negative Breast Cancer, Keytruda with Chemotherapy improved median Overall Survival to 23 months compared to 16.1 months with Chemotherapy alone.
Role in Cancer Treatment
As can be seen in the above clinical trials, Checkpoint Inhibitors represent a distinct improvement over Chemotherapy with the added benefit of reduced side effects. In various Checkpoint Inhibitor studies, anywhere from 15 – 40% of patients achieve long term survival by periodically receiving Checkpoint Inhibitor Therapy.
However, as also can be seen, Checkpoint Inhibitors are not enough for the majority of patients to achieve long term survival in advanced or aggressive cancers. For these patients, Checkpoint Inhibitors may not be the right solution or at a minimum can be a great adjuvant therapy to complement more advanced Immunotherapies like Immunocine’s Treatment.
Also as a final word of caution to patients considering Checkpoint Inhibitors, some patients have experienced Hyperprogression, which is accelerated cancer growth due to Checkpoint Inhibitor treatment. In one study comparing a variety of cancer types, 9% of patients demonstrated Hyperprogression caused by Checkpoint Inhibitor Therapy.
Advantages of Checkpoint Inhibitors
More effective than Chemotherapy for a variety of cancer types
Off the shelf medication that is easily administered orally or intravenously
Reduced side effects when compared with Chemotherapy
Easily included in a larger treatment protocol
Disadvantages of Checkpoint Inhibitors
Often taken for a prolonged or indefinite amount of time
Can actually cause rapid cancer progression for a subset of patients
Useless if the tumors or immune cells don’t express this specific checkpoint
Rarely develops immunological memory to fight relapses
References
Barrueto, L et al. Resistance to Checkpoint Inhibitors in Cancer Immunotherapy. Transl Oncol. 2020. PMID: 32114384
Johnson, D et al. Immune-Checkpoint Inhibitors: Long-term Implications of Toxicity. Nature. 2022. PMID: 35082367
Jenkins, R et al. Mechanisms of Resistance to Immune Checkpoint Inhibitors. Br J Cancer. PMID: 29319049
Brahmer JR, Lee JS, Ciuleanu TE, et al. Five-year survival outcomes with nivolumab (NIVO) plus ipilimumab (IPI) versus chemotherapy (chemo) as first-line (1L) treatment for metastatic non–small cell lung cancer (NSCLC): Results from CheckMate 227. J Clin Oncol.
Cortes J, et al; Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N Engl J Med. PMID: 35857659.
Champiat S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated Clin Cancer Res. (2017)
READ THIS NEXT
Dr. Nasha Winters’ Mission to Create a New Standard of Cancer Treatment
For decades, Dr. Nasha Winters has been a dynamic force in the field of integrative oncology with an emphasis on the metabolic approach to c
Read MoreWhy Cancer May Stop Responding to Treatment — The Importance of Adaptive Cancer Therapies
Cancer is a complex and challenging disease to treat. While many cancers initially respond well to treatment, it’s not uncommon for th
Read MoreThe Unsung Heroes: Dendritic Cells in the Battle Against Pancreatic Cancer
Pancreatic cancer stands as one of the most formidable adversaries in the realm of oncology. This silent killer often evades early detection
Read More