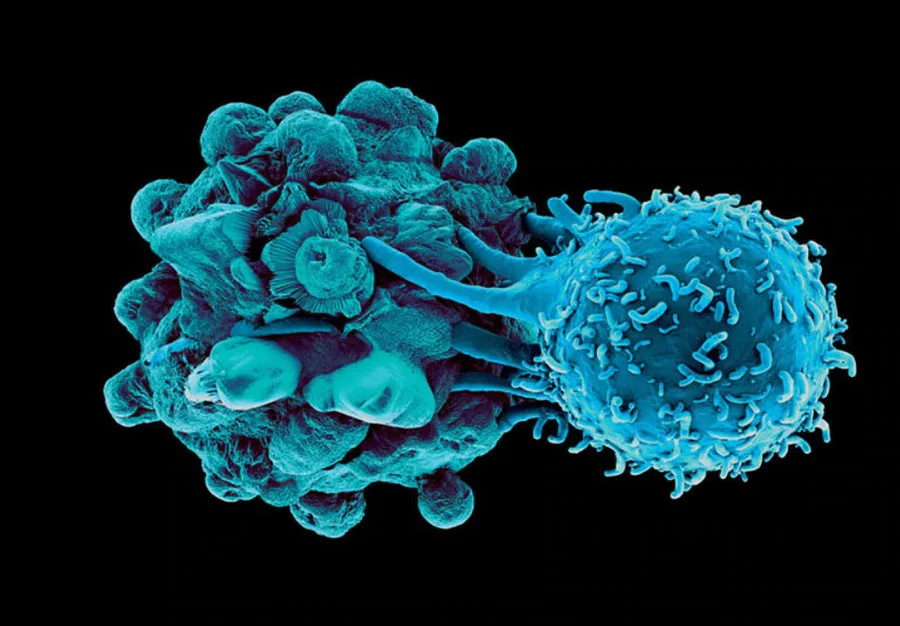
The Unsung Heroes: Dendritic Cells in the Battle Against Pancreatic Cancer

Pancreatic cancer stands as one of the most formidable adversaries in the realm of oncology. This silent killer often evades early detection and metastasizes rapidly, rendering conventional treatments less effective1. However, amidst the daunting landscape of pancreatic cancer research, there shines a beacon of hope – dendritic cells. These unsung heroes of the immune system have been increasingly recognized for their pivotal role in combating pancreatic cancer and offering novel therapeutic avenues2. Let’s delve into the intricacies of pancreatic cancer, the dysfunction of dendritic cells within this context, and the promising potential they hold as a therapeutic intervention.
Understanding Pancreatic Cancer
Pancreatic cancer arises when malignant cells form in the tissues of the pancreas, an organ located behind the stomach. Despite accounting for only 3% of all cancer diagnoses in the United States, pancreatic cancer ranks as the fourth leading cause of cancer-related deaths. The American Cancer Society estimates that in 2024, over 60,000 individuals in the United States will receive a diagnosis of pancreatic cancer, with approximately 48,000 succumbing to the disease. The dire prognosis associated with pancreatic cancer stems from its aggressive nature, with a five-year survival rate hovering around a meager 10%3.
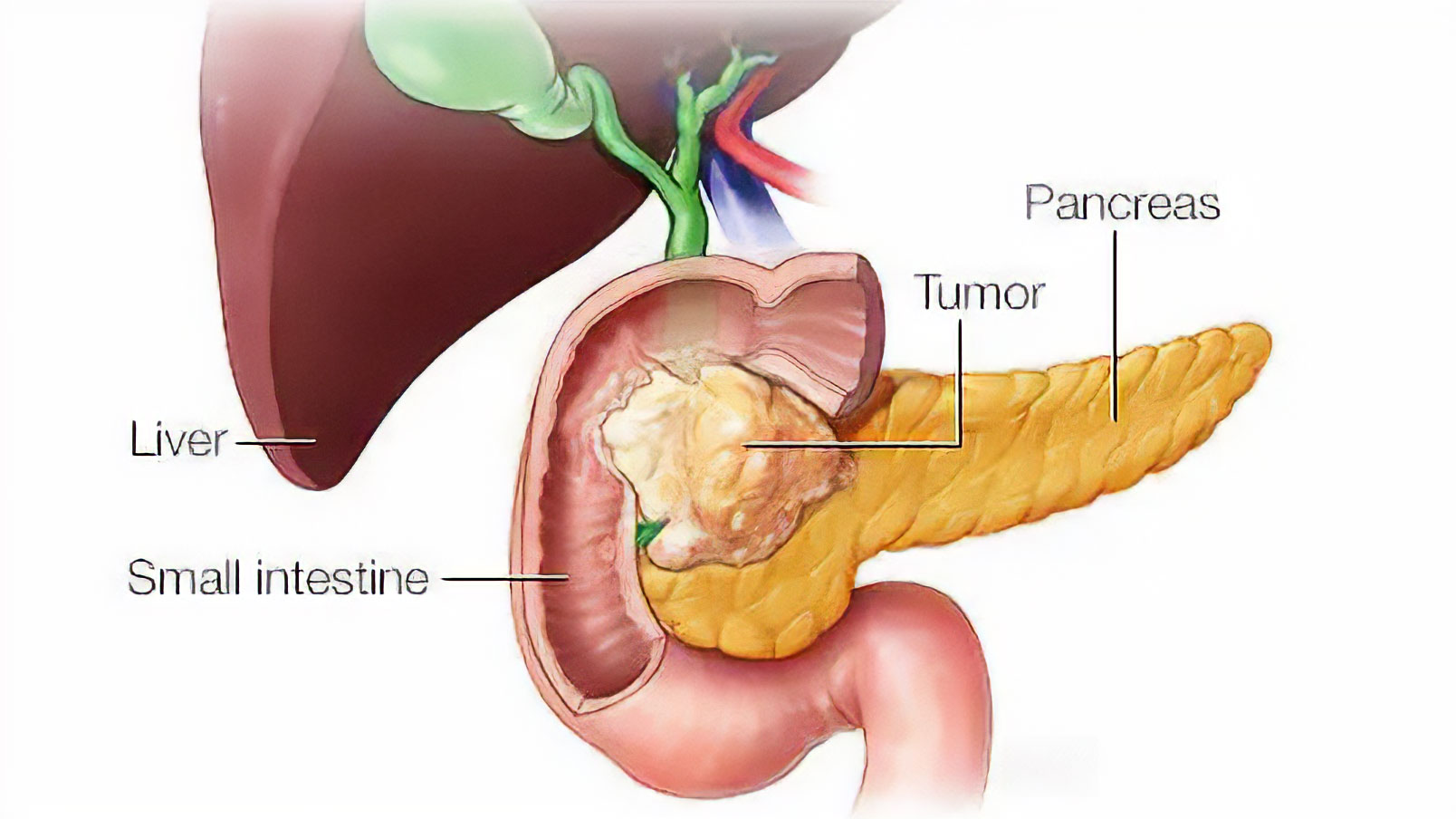
The Dendritic Cell Dilemma
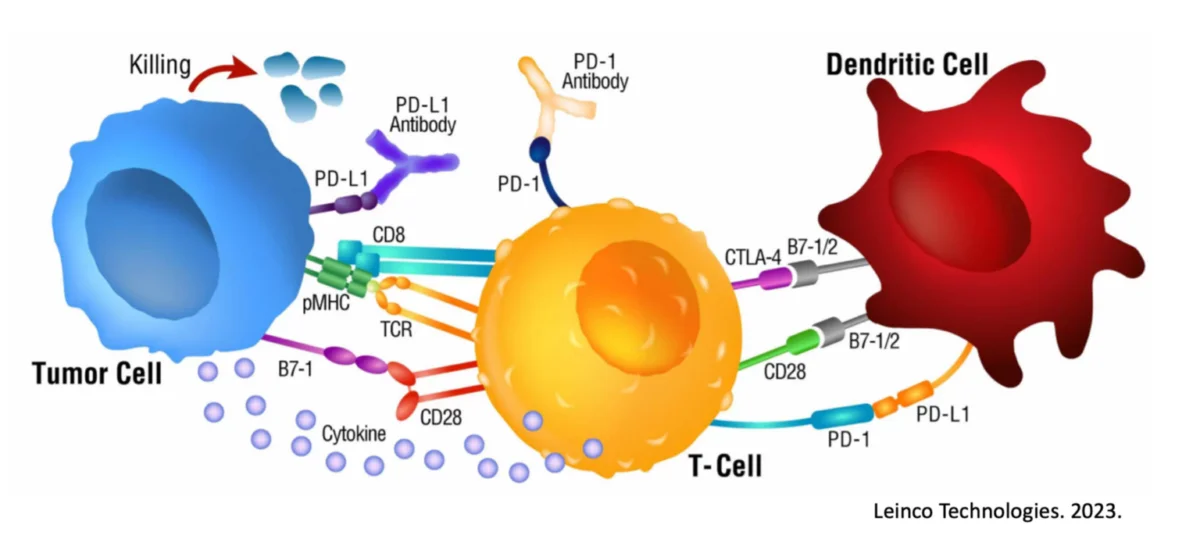
Dendritic cells (DCs) serve as the sentinels of the immune system, orchestrating the body’s defense against foreign invaders, including cancer cells4. However, in the context of pancreatic cancer, research suggests that these guardian cells may become dysfunctional, contributing to disease progression5. Studies have revealed alterations in the phenotype and function of dendritic cells within the tumor microenvironment, impairing their ability to mount an effective anti-tumor immune response.
For example, it was recently shown that one of the reasons there is a diminished immunotherapeutic ability of T-cells in pancreatic ductal adenocarcinoma (PDAC) is because of the scarcity of functional dendritic cells in the pancreas5. It is important to note though that while it has been shown that antigen-experienced dendritic cells can promote tumor rejection6, the mere presence of dendritic cells is not enough to prompt clinically meaningful results5.

And though in the past this dysfunction of dendritic cells was thought to potentially be a byproduct of tumorigenesis, it has now been shown to be one of the earliest events, helping to promote carcinogenesis itself7. As the cancer continues to evolve and expand, dendritic cell abundance and functionability steadily decline. To make matters worse, dendritic cells missing the correct cues can then be converted into tumor-promoting cells which prompt the development of immunosuppressive regulatory T cells to help protect the growing cancer8.
On the flip side, the increased percentage of proper dendritic cells within a patient (even those considered unresectable) has been directly correlated to improved prognoses9. In line with these findings, others have found that the cytokine milieu secreted from immune-promoting dendritic cells can have a significant impact on patient outcomes10.
With the apparent importance of dendritic cells in responses and outcomes to pancreatic cancer, the question becomes whether or not we can use dendritic cells to our advantage.

Leveraging Dendritic Cells as a Therapeutic Intervention
The dysregulation of dendritic cells within the tumor microenvironment presents a compelling rationale for exploring dendritic cell-based therapies in pancreatic cancer. Dendritic cell-based immunotherapies aim to harness the inherent antigen-presenting capabilities of dendritic cells to stimulate a robust anti-tumor immune response.
A recent trial of 134 patients demonstrated a statistically significant increase in survival metrics when provided an antigen-loaded dendritic cell vaccine11, and side effects were minimal. Another trial was able to show that cancer lysate loaded dendritic cells did indeed upregulate responding T cells and activation levels, and 70% of patients had not experienced disease recurrence or progression at a follow-up of 25 months12.

Another important mechanistic study revealed that the use of antigen-loaded dendritic cells was able to rewrite the tumor transcriptome and mitigate the expression of inhibitory receptors designed to promote tumor growth13. A 2023 review further summarized the importance of providing the dendritic cells the correct signals and targeting information for the ultimate outcome of the patient14.
And perhaps most impressively is that 7 out of 8 PDAC patients (as of March 2024) that have received double-loaded dendritic cells are still in complete remission (some past 3 years) and with perfect or near-perfect quality of life scores15. This trial is still open at Baylor College of Medicine in Houston, Tx, and continues to utilize the Immunocine technology.
The Immunocine Edge with Dendritic Cell Biology
There ultimately should be 3 take aways from this piece:
- Dendritic Cells are dysfunctional early and often in cases of pancreatic cancer and directly correlated with outcomes.
- Dendritic Cell therapies could be useful in combatting pancreatic cancer and restoring semblance of normalcy to the patient’s immune system.
- It is not enough to just use dendritic cells, but to use dendritic cells correctly.

This is where Immunocine comes into play. The Immunocine foundation is not “just to use dendritic cells,” but rather to use them correctly. It may seem arrogant to suggest others are not using these cells optimally, but that is what the data say16, 17, 17, 19. By missing the importance of ‘double loading’ a patient’s dendritic cells in a very precise manner, numerous critical activators are missing. Additionally, the true breadth of the neoantigen expression of the cancer is not encapsulated by the dendritic cells, and the responding immune response is subpar20, 21. And this is more than just theoretical for pancreatic cancer, as this technology has been demonstrated to be superior in creating long lived, durable immune responses capable of eliminating human pancreatic cancers22, and is currently proving much success in clinical trials in the US.
The pivotal role of dendritic cells in the fight against pancreatic cancer cannot be overstated. While their dysfunction presents a significant challenge, their potential as therapeutic agents offer a ray of hope for patients. However, it is crucial to recognize that harnessing dendritic cells effectively is paramount, and this is where Immunocine’s approach stands out. Immunocine’s focus on optimizing dendritic cell therapy through precise loading and activation techniques represents a paradigm shift in the field. By acknowledging the importance of maximizing dendritic cell functionality, Immunocine not only addresses the shortcomings of traditional approaches but also offers a promising pathway towards improved patient outcomes and the eventual conquest of pancreatic cancer.
References
- Park, W., Chawla, A. & O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 326, 851-862 (2021).
- Galati, D. & Zanotta, S. Dendritic Cell and Cancer Therapy. Int J Mol Sci 24 (2023).
- SEER. Cancer Stat Facts: Pancreatic Cancer. 2023 [cited]Available from: https://seer.cancer.gov/statfacts/html/pancreas.html
- Spiering, M.J. Primer on the Immune System. Alcohol Res 37, 171-175 (2015).
- Hegde, S. et al. Dendritic Cell Paucity Leads to Dysfunctional Immune Surveillance in Pancreatic Cancer. Cancer Cell 37, 289-307 e289 (2020).
- Deicher, A. et al. Targeting dendritic cells in pancreatic ductal adenocarcinoma. Cancer Cell Int 18, 85 (2018).
- Lin, J.H. et al. Type 1 conventional dendritic cells are systemically dysregulated early in pancreatic carcinogenesis. J Exp Med 217 (2020).
- Barilla, R.M. et al. Specialized dendritic cells induce tumor-promoting IL-10(+)IL-17(+) FoxP3(neg) regulatory CD4(+) T cells in pancreatic carcinoma. Nat Commun10, 1424 (2019).
- Hirooka, S. et al. The role of circulating dendritic cells in patients with unresectable pancreatic cancer. Anticancer Res 31, 3827-3834 (2011).
- James, C.A. et al. Systemic Alterations in Type-2 Conventional Dendritic Cells Lead to Impaired Tumor Immunity in Pancreatic Cancer. Cancer Immunol Res11, 1055-1067 (2023).
- Gansauge, F., Poch, B., Kleef, R. & Schwarz, M. Effectivity of long antigen exposition dendritic cell therapy (LANEXDC(R)) in the palliative treatment of pancreatic cancer. Curr Med Chem 20,4827-4835 (2013).
- Lau, S.P. et al. Autologous dendritic cells pulsed with allogeneic tumour cell lysate induce tumour-reactive T-cell responses in patients with pancreatic cancer: A phase I study. Eur J Cancer 169, 20-31 (2022).
- Lau, S.P. et al. Dendritic cell vaccination and CD40-agonist combination therapy licenses T cell-dependent antitumor immunity in a pancreatic carcinoma murine model. J Immunother Cancer 8 (2020).
- Zhang, X., Xu, Z., Dai, X., Zhang, X. & Wang, X. Research progress of neoantigen-based dendritic cell vaccines in pancreatic cancer. Front Immunol 14, 1104860 (2023).
- Medicine, B.C.o. Th-1 Dendritic Cell Immunotherapy Plus Standard Chemotherapy for Pancreatic Adenocarcinoma (DECIST). 2024 [cited]Available from: https://clinicaltrials.gov/study/NCT04157127?cond=pancreatic%20cancer&term=th1&rank=2
- Decker, W.K. et al. Deficient T(H)-1 responses from TNF-alpha-matured and alpha-CD40-matured dendritic cells. J Immunother 31, 157-165 (2008).
- Decker, W.K. et al. Th-1 polarization is regulated by dendritic-cell comparison of MHC class I and class II antigens. Blood 113, 4213-4223 (2009).
- Halpert, M.M. et al. MHC class I and II peptide homology regulates the cellular immune response. FASEB J 34, 8082-8101 (2020).
- Liang, D. et al. AIMp1 Potentiates T(H)1 Polarization and Is Critical for Effective Antitumor and Antiviral Immunity. Front Immunol 8, 1801 (2017).
- Konduri, V. et al. A subset of cytotoxic effector memory T cells enhances CAR T cell efficacy in a model of pancreatic ductal adenocarcinoma. Sci Transl Med13 (2021).
- Konduri, V. et al. CD8(+)CD161(+) T-Cells: Cytotoxic Memory Cells With High Therapeutic Potential. Front Immunol 11, 613204 (2020).
- Konduri, V. et al. Chemo-immunotherapy mediates durable cure of orthotopic Kras(G12D)/p53(-/-) pancreatic ductal adenocarcinoma. Oncoimmunology 5,e1213933 (2016).
READ THIS NEXT
Dr. Nasha Winters’ Mission to Create a New Standard of Cancer Treatment
For decades, Dr. Nasha Winters has been a dynamic force in the field of integrative oncology with an emphasis on the metabolic approach to c
Read MoreWhy Cancer May Stop Responding to Treatment — The Importance of Adaptive Cancer Therapies
Cancer is a complex and challenging disease to treat. While many cancers initially respond well to treatment, it’s not uncommon for th
Read MoreInflammation in Cancer
In today’s world, chronic inflammation is an ever-present challenge linked to serious diseases, notably cancer. However, the pa
Read More