Why don’t Checkpoint Inhibitors Work More Often?
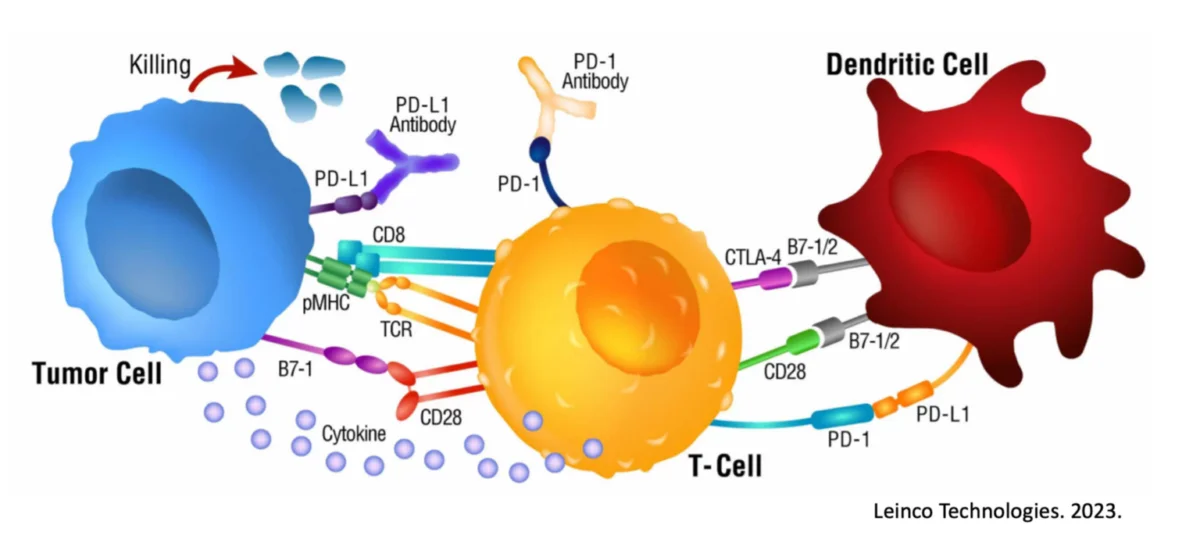
With immunotherapy really taking off in the past decade, many cancer patients are rightfully interested in adding this potential modality to their treatment plan. After all, it has long been established that the immune system can eliminate cancer if properly educated1, 2 and powered3, and that this is not dependent on where or what started the cancer. For solid cancers, the number one immunotherapy option is currently some form of a checkpoint inhibitor (ICI), such as:
PD-1 Inhibitors
Keytruda (Pembrolizumab)
Opdivo (Nivolumab)
Libtayo (Cemiplimab)
CTLA-4 Inhibitors
Yervoy (Ipilimumab)
Imjuno (Tremelimumuab)
PD-L1 Inhibitors
Tecentriq (Atezolizumab)
Imfinzi (Durvalumab)
Bavencio (Avelumab)
LAG-3 Inhibitor + PD-1 Inhibitor
Opdualag (Relatlimab + Nivolumab)
And while these new options are exciting, they do not work for everyone. In fact, they do not work for most, with an estimated 43.6% of cancer patients in the US eligible for such a drug and only 12.5% of patients responding to it4. This even includes a relatively underwhelming 20% response rate in melanoma cases, a cancer thought to be extremely immunogenic and amenable to immunotherapy5, 6. More recently, some publications have pointed out the failure of the FDA to honor its commitment to remove such drugs from certain market indications after a lack of confirmatory evidence post an accelerated approval7. Even worse, it now looks like up to 9% of US cancer patients may be exposed to ICIs that have negative phase 3 trial results, which lowers the estimated response rate from 12.5% to 10.9%8.
Given that all these drugs are designed to do is to empower immune cells, one has to question why they are not more successful and how we may be able to improve the odds.
How do Checkpoint inhibitors Work?
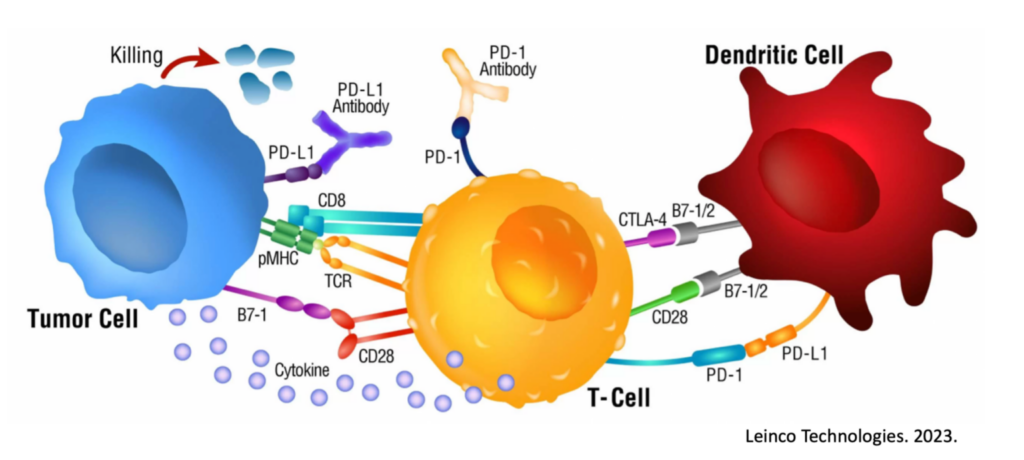
To begin, we must understand the basic premise for how a checkpoint inhibitor works. Immune cells, such as Killer T cells, have “checkpoints” or “brakes” on their surface such as PD-1 and CTLA-4 which are used within homeostatic conditions to essentially prevent autoimmunity and widespread inflammatory conditions9. After all, an immune response to eliminate a target is valuable and important. An immune response that continues when not needed or off-target is a problem.
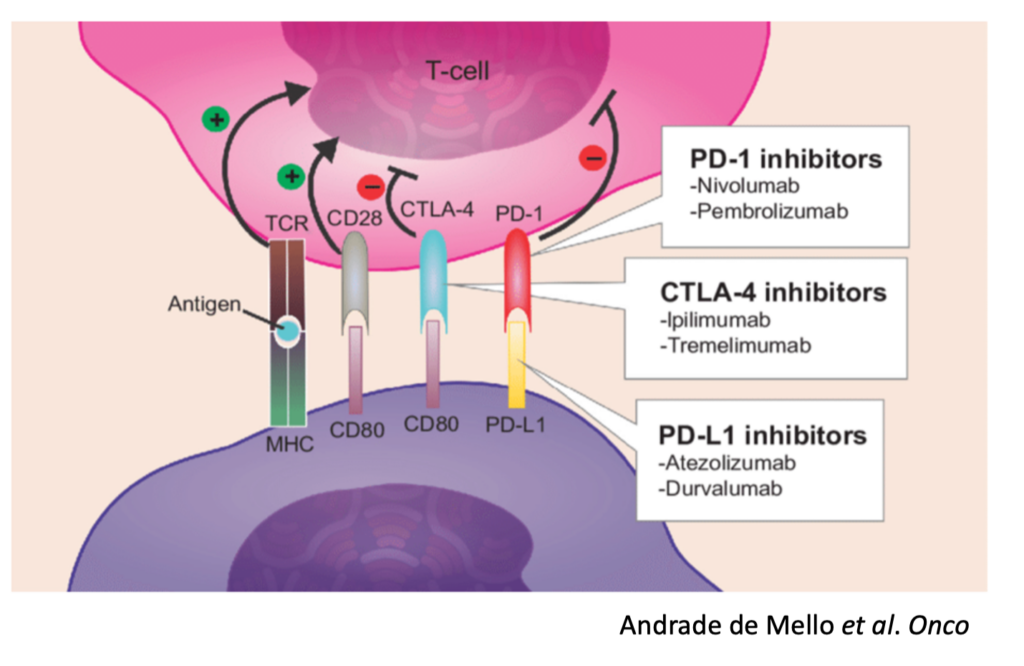
Unfortunately, many cancers have seemingly learned how to exploit these “checkpoints” on immune cells and can hijack them to reduce and even eliminate a cancer-specific immune response. For example, some tumors express PD-L1, a ligand (“L”) or binder, for PD-1 on Killer T cells. In this scenario, the Killer T cells show up at the tumor to destroy it, and the tumor simply signals the T cell through the PD-L1 / PD-1 interaction to turn it off.
Checkpoint inhibitors were created and are used to interfere with this cancer-hijacking ability to turn off the responding immune response.
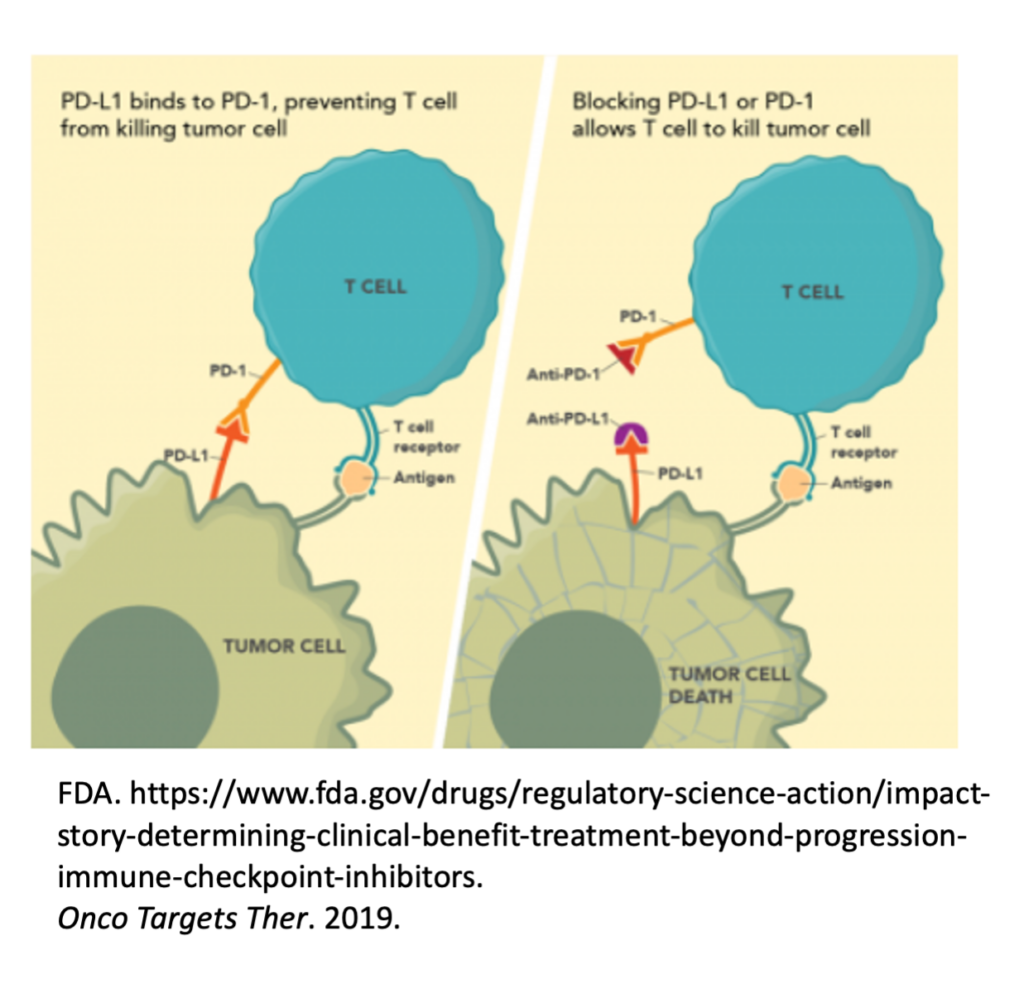
Checkpoints Are Not Always Available for Pharmaceutical Intervention
At this point though, it starts to become more obvious what the rate-limiting step is regarding ICI functionality. ICI’s will predominantly work to empower / repower an already activated and targeted immune response, meaning in the absence of another immunotherapy, an immune response needs to be spontaneously launched first. It is important to know that checkpoints like PD-1 are expressed after activation of the immune cell in a feedback-loop manner, but not before10, 11, 12, 13. In other words, these checkpoints serve as immune activation markers and are not ideal pharmaceutical targets before an immune response is created.
Unfortunately, the current rate of spontaneous immune response (and tumor regression) across the board is estimated to be between 1 in 60,000 to 100,00014, 15, 16. Even in cancers considered prone to spontaneous regression, the numbers are still woefully unimpressive – such as 10% for melanoma17, and 1 in 400 if it has metastasized18, 19. In the absence of an already ongoing immune response, there isn’t much an ICI can do, except cause side effects and empower immune cells to randomly attack non-cancerous targets20.
So how do we fix this?
Immunocine Paves the Way
This is where Immunocine not only offers a robust, personalized, physiological immune response in and of itself, but also greatly improves the odds of responding to ICI’s. Immunocine focuses on creating and deploying a truly unique, cutting-edge Dendritic Cell therapy (which can be further studied a variety of ways, including this quick post here). This immunotherapy product is made from the patient’s own tumor and own immune cells, and then reinserted near functional lymph nodes draining the tumor antigens. After decades of studies, publications, and clinical trials, it seems there is better than a 75% chance of successfully reprogramming a patient’s immune system after this process to finally recognize and attack the cancer.
And with that activation and targeted immune response, checkpoints are eventually likely to become expressed.
While some patients will need no other intervention than the Immunocine Dendritic Cell technology, other patients may find benefit in adding treatment options. One such addition that may strongly garner attention is the addition of a checkpoint inhibitor, such as Keytruda, a couple of months after finishing the Immunocine treatment. At that point, it is likely that the Immunocine Dendritic Cells have done their job and launched a targeted and strong anti-cancer immune response (a.k.a. Immune response launched – check). Whether or not it is strong enough on its own will often reduce to many personal variables, including tumor burden and stage, immune strength within the person, previous treatment attempts, and more. However, the subsequent addition of one of these drugs may greatly speed up a response and/or overcome the tumor’s attempts to reduce the attack.
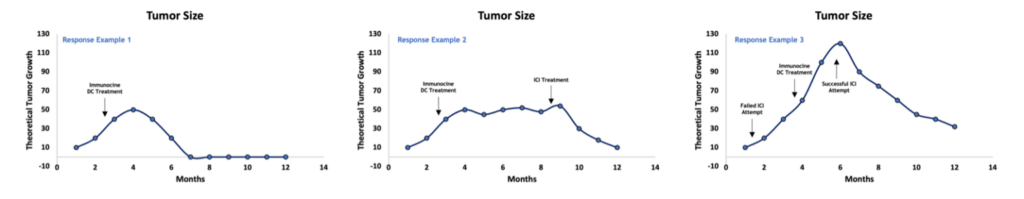
Immunocine Adds Treatment Response And Treatment Options
One last thing to mention – many patients are told that “Immunotherapy” or “Checkpoint inhibitors” will not work for them. It is important to understand the basis of that diagnoses. If a patient’s tumor is analyzed and is not positive for PD-L1, and there is no evidence of an underlying immune response trying to fight the cancer, it stands to reason that an ICI (targeting PD-1 or PD-L1) wouldn’t do much here. There is no checkpoint to protect, no immune response to empower, and no hijacking the tumor is trying to utilize. Without more to go on, it would be a waste of time and resources for a patient to undergo ICI treatment within these parameters. But after the Immunocine treatment, which is likely to launch a coordinated immune response, suddenly checkpoints such as PD-1 on T cells become ‘in play.’
Checkpoint inhibitors are truly revolutionary, and our goal is to say nothing contrary to that. However, they are significantly more likely to be successful if introduced after a targeted immune response has been launched against the cancer. If you are not one of the lucky people to spontaneously generate that, then look to Immunocine for a proper and physiological reprogramming so that your body starts working for you and these other beneficial options come to the table.
To take the first step to getting your immune system off the sideline and into the game, please visit us here.
References
1. Messerschmidt, J.L., Prendergast, G.C. & Messerschmidt, G.L. How Cancers Escape Immune Destruction and Mechanisms of Action for the New Significantly Active Immune Therapies: Helping Nonimmunologists Decipher Recent Advances. Oncologist 21, 233-243 (2016).
2. Knuth, A., Danowski, B., Oettgen, H.F. & Old, L.J. T-cell-mediated cytotoxicity against autologous malignant melanoma: analysis with interleukin 2-dependent T-cell cultures. Proc Natl Acad Sci U S A 81, 3511-3515 (1984).
3. Papac, R.J. Spontaneous regression of cancer: possible mechanisms. In Vivo 12, 571-578 (1998).
4. Haslam, A. & Prasad, V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw Open 2, e192535 (2019).
5. Jessurun, C.A.C., Vos, J.A.M., Limpens, J. & Luiten, R.M. Biomarkers for Response of Melanoma Patients to Immune Checkpoint Inhibitors: A Systematic Review. Front Oncol 7, 233 (2017).
6. Knight, A., Karapetyan, L. & Kirkwood, J.M. Immunotherapy in Melanoma: Recent Advances and Future Directions. Cancers (Basel) 15 (2023).
7. Gill, J. & Prasad, V. A reality check of the accelerated approval of immune-checkpoint inhibitors. Nat Rev Clin Oncol 16, 656-658 (2019).
8. Haslam, A., Gill, J. & Prasad, V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for Immune Checkpoint Inhibitor Drugs. JAMA Netw Open 3, e200423 (2020).
9. Buchbinder, E.I. & Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol 39, 98-106 (2016).
10. Chikuma, S. et al. PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J Immunol 182, 6682-6689 (2009).
11. Simon, S. & Labarriere, N. PD-1 expression on tumor-specific T cells: Friend or foe for immunotherapy? Oncoimmunology 7, e1364828 (2017).
12. Hong, J.J., Amancha, P.K., Rogers, K., Ansari, A.A. & Villinger, F. Re-evaluation of PD-1 expression by T cells as a marker for immune exhaustion during SIV infection. PLoS One 8, e60186 (2013).
13. Jubel, J.M., Barbati, Z.R., Burger, C., Wirtz, D.C. & Schildberg, F.A. The Role of PD-1 in Acute and Chronic Infection. Front Immunol 11, 487 (2020).
14. Cole, W.H. & Everson, T.C. Spontaneous regression of cancer: preliminary report. Ann Surg 144, 366-383 (1956).
15. Dobosz, P. & Dzieciatkowski, T. The Intriguing History of Cancer Immunotherapy. Front Immunol 10, 2965 (2019).
16. Jessy, T. Immunity over inability: The spontaneous regression of cancer. J Nat Sci Biol Med 2, 43-49 (2011).
17. Aung, P.P., Nagarajan, P. & Prieto, V.G. Regression in primary cutaneous melanoma: etiopathogenesis and clinical significance. Lab Invest 97, 657-668 (2017).
18. Tran, T., Burt, D., Eapen, L. & Keller, O.R. Spontaneous regression of metastatic melanoma after inoculation with tetanus-diphtheria-pertussis vaccine. Curr Oncol 20, e270-273 (2013).
19. Radha, G. & Lopus, M. The spontaneous remission of cancer: Current insights and therapeutic significance. Transl Oncol 14, 101166 (2021).
20. Martins, F. et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 16, 563-580 (2019).
READ THIS NEXT
Metastatic Melanoma: A Deep Dive into the Aggressive Form of Skin Cancer
Melanoma, a type of skin cancer, can be a frightening diagnosis. But within the spectrum of melanoma, metastatic melanoma stands out as part
Read MoreImmunotherapy vs. Chemotherapy Side Effects: Comparing the Objectives
On August 4th, we posted an overview of how chemotherapeutic side effects differ from those experienced during immunotherapy. Informative, b
Read MoreTriumph Over Stage 4 Melanoma: Brock’s Inspiring Journey
Brock had his world turned upside down when he was diagnosed with Stage 4 Melanoma in July 2022. With his cancer growing by 300% within five
Read More